Introduction
With over 611.7 million hashtag views on TikTok,1 fasting and intermittent fasting is certainly well-known within the public sphere. But just how famous are fasting and its effects within the scientific and clinical realms? Aside from its popularity as a weight loss tactic, fasting has recently gained traction among researchers and clinicians as a potential natural intervention for IBD and other inflammatory conditions. Although the most touted dietary regimens have not always been found to be the most healthy, this emerging trend could flip the switch. Read on to learn about the five most common types of fasting, what the research says about the benefits and risks, and whether or not the evidence suggests any benefits of fasting for IBD.
What is fasting?
Broadly, fasting refers to a dietary restriction strategy involving reduced nutrient consumption for a specified time interval.2 Though an increasingly popular trend in the niche of “healthy lifestyles”, fasting is nothing new to humans and appears in several of the world’s oldest major religious traditions, including Ramadan, Yom Kippur, Lent, and Navaratri. While the historical motivations of fasting may include the spiritual, it turns out there are a number of potential health benefits as well to these periods of reduced eating.
Importantly, fasting (a dietary restriction-based strategy) isn’t inherently the same as caloric restriction (consuming fewer calories than you expend; typically 20-40% of normal caloric intake). While fasting may include an overall reduction in calorie consumption, caloric restriction is not the main priority of fasting; instead, fasting regimens—like intermitted fasting (IF) and periodic fasting (PF)—can focus on reduced intake of a specific dietary component (generally macronutrients like protein, fat, or carbohydrates) or specific periods of time for reduced nutrient intake.
Caloric restriction on its own has been demonstrated to improve some age-related changes and immune processes3–5 as well as reduce the risk of several conditions like diabetes, cancer, and cardiovascular disease.3,6,7 However, when caloric restriction is implemented on a constantly recurring and/or persistent basis, all those missing calories can start to add up to extremely low body mass index (BMI)3,6,7 or diminished immune function.3,8,9 The body needs fuel to work! Therefore, periods of fasting can confer some of the benefits of reduced nutrient intake while mitigating some of the harmful side effects of overapplying caloric restriction.
The proper functioning of the brain and other active tissues (such as blood cells) is fueled entirely by glucose (sugar), so glucose must always be readily available in the bloodstream for a person to survive. Accordingly, glucose levels primarily determine the body’s nutrient state. Fasting decreases blood glucose levels in the blood, which thereby alters the nutrient state of the body. This in turn modifies the release of metabolic hormones from the liver to normalize blood glucose levels, such as glucagon (increases blood glucose levels) and insulin (decreases blood glucose levels). In summary, the nutrient state dictates hormone release from the liver to provide the brain and other active tissues with the fuel they need in states of altered nutrient intake.3,10,11 Thus, fasting derives its benefits from metabolic changes to the nutrient state (aka glucose levels) and is ultimately driven by the brain’s and other active tissues’ energy needs.
Common Types of Fasting
There is a huge range of what people mean when they use the term “fasting”, and in truth, there is no one “right” way to go about it! Dietary strategies of any kind are deeply individual to a person’s metabolic needs and access to different foods. Fasting strategies generally fall into two categories: Intermittent Fasting (IF; shorter repeating intervals of fasting and eating) and Periodic Fasting (PF; longer intervals of fasting paired with unrestricted eating intervals; can be either repeated or done as needed).3 Below is a breakdown of the five most common types of fasting3,12 to help you and your healthcare team determine if, and which type of, fasting might be appropriate for you to explore. It is important to note that although there have certainly been some promising evidence presented supporting the benefits of fasting, this research remains in its infancy and should only be started following approval by and under the supervision of your healthcare professionals (more on this later).
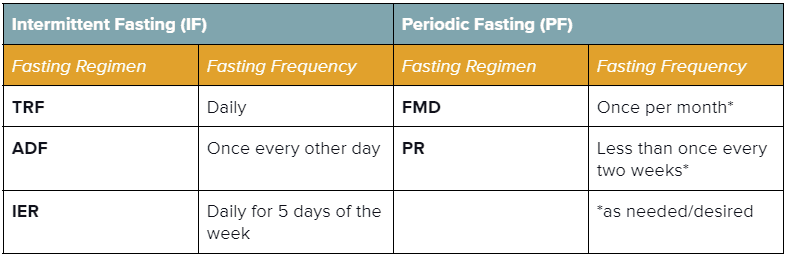
| Intermittent Fasting (IF)3,12 Alternating between intervals of short fasting or reduced nutrient intake (16-48 hours) and eating (8-120 hours).3,12 | Periodic Fasting (PF)3Either entirely restricting (‘water-only) or drastically reducing caloric intake (‘FMD’) for a prolonged time (>2 days; ~2-7 days); accompanied by an ad-libitum (unrestricted) refeeding interval3 |
|---|---|
| Time-Restricted Feeding / Fasting (TRF)Designating a restricted time interval (typically ≤12 hours) each day for eating. Sometimes referred to as ratios (e.g., a 16:8 fast is 16 hours of fasting & 8 hours of eating per day). This type of fasting is common in religious practices like Ramadan and Tzom Gedalia.3 | Fasting-Mimicking Diet (FMD)A monthly interval of reduced (30-50% of normal) caloric intake for ~4-7 successive days, accompanied by an ad-libitum (unrestricted) refeeding interval (generally 10-25 days). This type of fasting is typically employed as a plant-based diet comprised of foods with low protein, low carbohydrate, and high unsaturated fat.3 |
| Alternate-Day Feeding / Fasting (ADF)Alternating between intervals of short fasting or reduced nutrient intake (16-48 hours) and eating (8-120 hours).3 | Prolonged Fasting (PR)An extremely calorie-restricted or water-only fasting interval (~2-7 days, sometimes up to 21 days), accompanied by an ad-libitum refeeding interval of 7+ days; may be done periodically or as needed (no more than once every two weeks).3 |
| Intermittent Energy Restriction (IER) Limiting energy intake (20-25% of daily energy needs) on designated fasting days (i.e., 5:2 diet, 5 non-fasting days & 2 fasting days).12 | NOTE: Periodic Fasting must be carried out under specialized medical supervision to prevent detrimental effects on health. |
Both intermittent fasting (IF) and periodic fasting (PF) have gained recent attention from the health and science communities due to their newly discovered potential benefits in improving longevity and healthspan.2,3 As a result, IF and PF have been proposed as promising therapeutic avenues for several inflammatory conditions, including IBD, psoriasis, and rheumatoid arthritis (RA).2,3,13
Proposed Benefits of Fasting
Because the essential idea of fasting—intervals of less nutrient intake and more nutrient intake—comes in varied flavors, each of the above strategies can elicit a range of physiological effects, and so the respective benefits and risks warrant individual consideration. In addition, fasting operates within the context of your own unique biology, so results can vary between individuals. Fasting is not a monolithic dietary intervention, and each study has taken a slightly different angle. These research variations—especially when combined with the current sparsity of data on individuals outside of what is considered the “general population”—can make results a challenge to compare and limits the research’s application in understudied populations. That being said, there are a few proposed benefits and risk considerations that are worth mentioning!
Why This Works: Fasting likely reduces inflammation by influencing different components of cellular pathways involved in inflammatory processes. For example, multiple studies have shown that IF (alternate-day fasting, 5:2, and TRF) and FMD are all associated with decreased C-Reactive Protein (CRP) levels – a protein that participates in the systemic inflammatory response.3,14–17 This supports previous findings that decreases in body fat—another effect of fasting—are associated with reduced levels of circulating CRP.19 Furthermore, a recent study18 that assessed serum markers in volunteers (N = 12 healthy adults) during fasted and fed states linked fasting to reduced numbers of circulating monocytes, monocyte metabolic activity, and inflammatory activity. Notably, this study also found fasting to improve inflammatory conditions without damaging antimicrobial immunity in mouse models.18
Why This Works: Studies23,24 performed in type 2 diabetic mouse models suggest that fasting may positively alter microbial diversity within the gut by increasing colonization by beneficial bacteria (such as particular species of Lactobacillus23,24, Oscillospira23, and Ruminococcus23) while decreasing colonization by bacterial strains known to be harmful at high abundances (such as certain species of Bacteroides23, Enterococcus24, and Streptococcus24).22,25–30 Mouse model studies of experimental colitis reported an increase in another beneficial gut microbe, Akkermansia muciniphila20,21, that may be involved in decreasing inflammation and improving epithelial barrier integrity.22 Fasting is correlated with increased SFCAs produced by A. muciniphilia in a microbiome study20 of two volunteer cohorts (one of younger males and one of middle-aged males) that performed IF for 1-month, and another experimental colitis mouse model study13 reported that fasting promotes intestinal stem cell regeneration (which improves epithelial barrier integrity), alleviates intestinal inflammation, and promotes the growth of beneficial gut microbe populations.
Why This Works: With IBDers at higher risk for the development of certain cancers (especially colorectal cancer32–36), this potential benefit is particularly relevant to the IBD community. Animal studies suggest that fasting may train cancer cells to become more sensitive to the metabolic changes induced by fasting, thereby creating a cellular environment protective against cancer cell growth and development.2,3,31,37–39 Moreover, fasting’s influence on the mTOR pathway (and subsequently its associated cellular processes of proliferation, autophagy, and apoptosis) also makes it a promising therapeutic candidate for cancer prevention and treatment.2,3,31
Why This Works: Animal models of cancer2,3,37,40,41,42(p),43, myocardial infarction44, diabetes45, stroke46,47, Alzheimer’s Disease (AD), Parkinson’s Disease (PD), and Huntington’s disease (HD)2,3,47–51 have demonstrated improved disease activity and enhanced functional outcomes in response to fasting.2,3 IF and PF can decrease risk factors such as levels of total cholesterol, triglycerides, fat mass, low-density lipoprotein (LDL), insulin resistance, leptin, systolic blood pressure, IGF-1, glucose, C-reactive protein (CRP).3,14–16 The influence of fasting on neurodegenerative processes is also likely owed to its role in stimulating autophagy, the body’s system of cellular waste disposal that reduces oxidative stress, the body’s system of cellular waste disposal that reduces oxidative stress.52–56 Inhibition of autophagy has been shown to cause neurodegeneration is tissue culture and animal studies.52–54,56–62 Genome-wide association studies have also implicated a role for disrupted autophagy in individuals with Crohn’s disease (CD).63–65
Why This Works: Animal and human studies have indicated that fasting can encourage cellular protection and repair and the removal of cellular debris and unhealthy cells.2,3,52,56,69 This influences cellular pathways (such as autophagy, apoptosis, and ketogenesis) involved in metabolism, nutrient-sensing, and stress resistance.2,3,52,56,69,70 Research has also demonstrated a protective effect of fasting in rodents against age-related conditions, such as cancers, heart disease, and neurodegeneration.2,3,12,47–51,70 In humans, fasting has been shown to decrease obesity, hypertension, asthma, and rheumatoid arthritis (RA).2,3,12,14,17,67,68 Thus, by triggering adaptive cellular responses (i.e., autophagy, apoptosis), fasting confers increased stress resistance, imbuing the body with an enhanced capacity to offset disease processes.2,3
Why This Works: In addition to improving longevity and healthspan, the enhancement of cellular protection can beneficially impact age-related changes. Aside from bolstering stress resistance pathways (mentioned above), clinical and epidemiological evidence suggests fasting can enhance cellular security by alleviating oxidative damage—another phenomenon that plays a critical role in age-related and disease-promoting processes.2,3 Fasting has also been shown to inhibit the mTOR pathway, which regulates cellular proliferation, autophagy, and apoptosis and is often upregulated in cancerous tumor cells.31
Why This Works: Animal studies and cell culture studies2,3,71–74,75(p) suggest that fasting may optimize energy metabolism by enhancing insulin sensitivity and lowering levels of blood pressure, body fat, IGF-1, glucose, atherogenic lipids, and inflammation—all of which are widely used as clinical parameters for the diagnosis of metabolic syndrome and can serve as indicators of overall metabolic health.76
Why This Works: By improving lipid profile, lowering blood pressure, and reducing oxidative stress, fasting may positively impact heart metabolism.67,77
Why This Works: The human body relies on three forms of energy metabolism: glycogen, lipid, and amino acid.11 After fasting for 12-24 hours, the body switches from breaking down glucose for energy to breaking down adipose (fat) tissue, thereby contributing to weight loss in the context of fasting.3,11 A recent review67 on the effects of IF on cardiometabolic health found that all types of IF fasting mentioned above (alternate-day fasting, the 5:2 diet, and TRF) in all studies analyzed were able to generate a clinically significant >5% weight loss and improve multiple metabolic parameters in obese individuals.67
As you can see, regulation of nutrient intake through periods of fasting can have wide-ranging effects across general human health in some of the trickiest conditions, especially in those that develop slowly over time as disruptions to normal immune function (such as in inflammatory conditions, cancer, and neurodegeneration). Can the numerous physiological benefits of intermittent fasting translate to meaningful benefits for people living with inflammatory bowel disease?
Fasting and IBD
While fasting has been associated with numerous benefits across a wide range of health concerns, four of the areas discussed above that are most relevant to IBDers include:
- Reducing inflammation.
- Reversing gut dysbiosis.
- Enhancing epithelial barrier integrity.
- Improving stress resistance and protection against chronic diseases like cancer.
IBD pathogenesis is characterized by dysbiosis of the gut microbiome and increased intestinal permeability, which fuel a runaway inflammatory response that can further exacerbate dysbiosis and damage to the intestinal barrier.78–81 Fasting poses a unique opportunity for disrupting this positive feedback loop based on the proposed benefits of improving gut microbiota composition, enhancing epithelial barrier integrity, and reducing inflammation.13,20–22,82,83 Together, tackling these three mechanisms of inflammatory bowel disease demonstrates that fasting may be a promising avenue to support healing and IBD symptom relief.
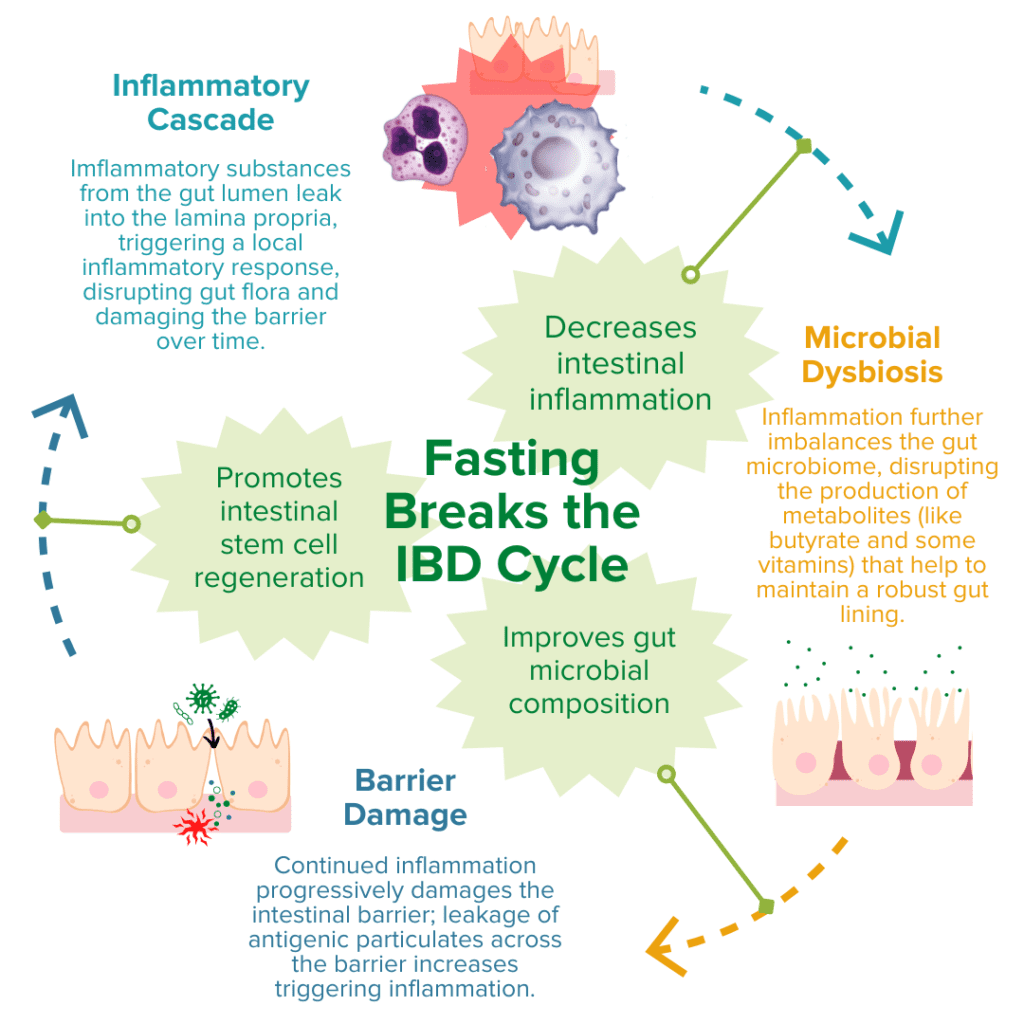
Production of SCFAs (such as butyrate, propionate, and acetate) by beneficial gut microbes (such as A. muciniphila) is involved in maintaining proper gut barrier function and regulation of intestinal inflammatory responses.13,20,21,84 For example, fasting has been shown to positively alter microbial diversity within the gut to promote the production of bacterial-derived SCFAs (such as propionate).20,21 These SCFAs can encourage the regeneration of intestinal cells and nourish the epithelial barrier, thereby enhancing gut barrier integrity, reducing leakage of antigenic (immune response-triggering) particulates from the gut lumen, and subsequently alleviating inflammation and preventing further damage to the barrier by immune cells.20,21,67,84 Another notable study recently reported that employing FMD cycles in a mouse model of colitis was able to ameliorate IBD-related pathologies by improving the microbial composition within the gut, decreasing intestinal inflammation, and promoting intestinal stem cell regeneration.13
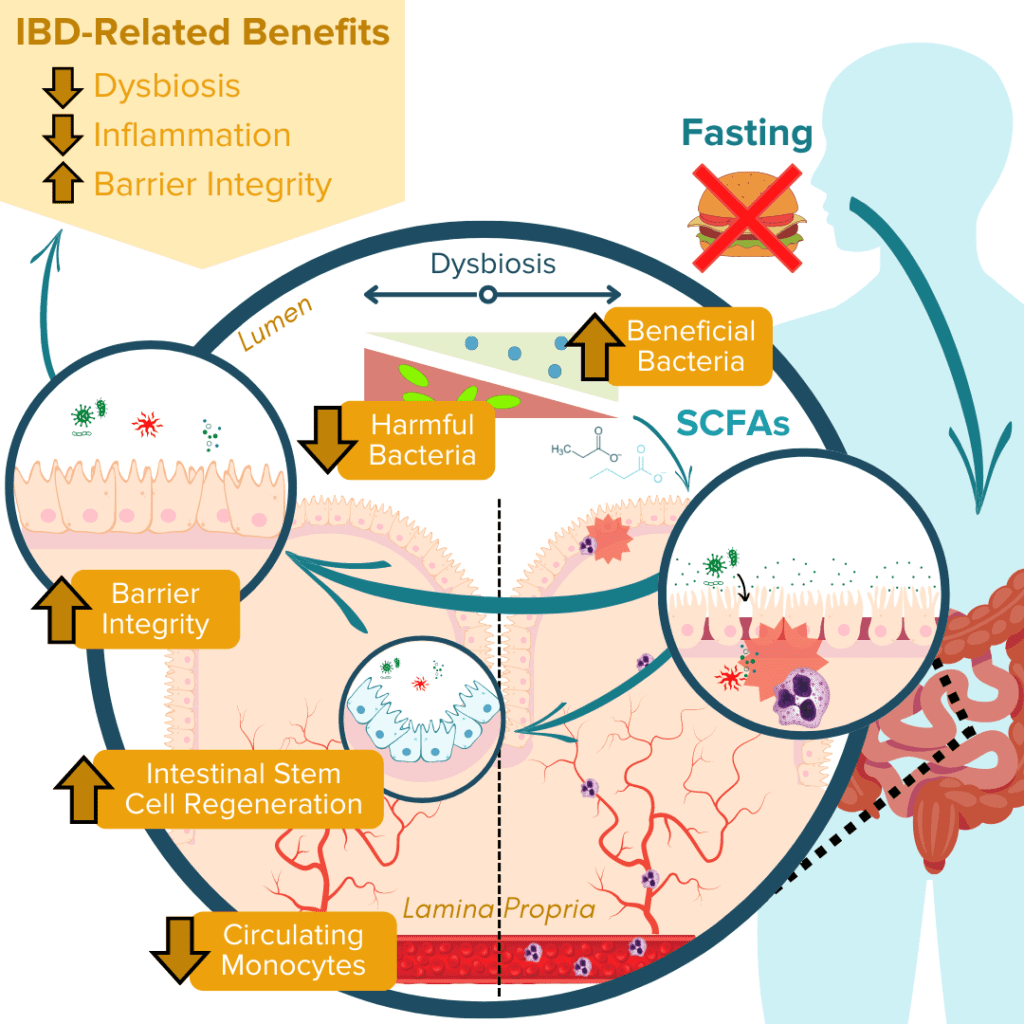
Ultimately, numerous animal studies have supported these beneficial effects of fasting on the three molecular mechanisms associated with IBD pathology. Human studies have also supported these findings. However, few of these studies have assessed these effects in individuals with clinically-diagnosed IBD, and so more research is needed to understand the direct impact of fasting on treatments and symptoms of ulcerative colitis and Crohn’s disease.2,3,11,12,83
Risks of Fasting
While fasting comes with a range of possible benefits for general health and IBD alike, as with any intervention, it is not without risk. Fasting causes a disruption, by design, to the nutrient state of the body. Because nutrient absorption is already a known issue in people with Crohn’s disease and ulcerative colitis, additional restriction of food intake can exacerbate malnutrition and lead to additional downstream effects on mood and stress. Specifically, poorly planned fasting regimens can lead to:
People with IBD are at higher risk for weight loss—especially during a flare89,90—due to intensified issues with nutrient absorption and associated diarrhea.91–93
This is one of clinicians’ primary concerns for IBDers86 because IBDers are already at high risk for malnourishment due to the physiological nature of IBD pathology (which decreases nutrient absorption and utilization due to widespread intestinal inflammation and damage)91. These features of IBD pathology also put IBDers at higher risk for micronutrient deficiencies2,3,12,85,86, so restricting nutrient intake by any means runs the risk of exacerbating current deficiencies.
The brain, blood, and nerve cells completely depend on glucose for energy.3,11 Restricting nutrient intake for prolonged intervals has the potential to severely decrease blood sugar levels, resulting in hypoglycemia. Hypoglycemia can lead to confusion and dizziness and potentially stimulate a nervous system response.94 Due to the disrupted nutrient absorption characteristic of IBD pathology, nutrients from the gut (such as glucose) are not properly absorbed, especially during a flare.89–91 Furthermore, recent research suggests that colonic inflammation (another hallmark of IBD) also plays a role in disrupting blood glucose levels.95,96 Accordingly, IBDers are at increased risk for abnormal blood glucose levels and development of diabetes.95–97
Headaches are a frequently cited side effect of fasting. Proposed mechanisms that may confoundingly contribute to the development of such headaches include hypoglycemia, dehydration, and caffeine withdrawal11,98,99. An additional study suggests that this fasting headache may involve the activation of a specific pro-inflammatory pathway involving the metabolism of eicosanoids (a type of signaling molecule made from fatty acid oxidation).11,100
A recent review12 on the metabolic effects of intermittent fasting identified four trials of modified fasting regimens in humans101–104 for which small percentages of participants (~15%) reported adverse effects of fasting on mood or other behavioral aspects, such as feeling cold, increased irritability, decreased energy, or hunger. However, results are somewhat mixed; mean improvements in mood were also reported in these studies, such as reduced tension, anger, and fatigue, and increased self-confidence and improved mood. Another study on the effects of a TRF schedule in young women (N=9) reported worsened mood, intensified irritability, challenges with concentration, heightened fatigue, eating-associated thoughts, fear of inability to adhere to the dietary restrictions, and excessive eating during refeeding periods.105,106 There have also been reports of extreme hunger from studies investigating the health effects of alternate-day fasting.12,107
In addition to the considerations noted above, a recent review70 published to guide physicians on fasting recommendations proposed that individuals who are under 18 and over 75, have a history of disordered eating, qualify as underweight status for Body Mass Index (BMI), are pregnant and/or breastfeeding, and/or have Diabetes Mellitus (type 2 diabetes) refrain from fasting. Thus, potentially more severe adverse effects may be possible in these populations.
Given these risks, it is all the more critical for people to be under the supervision of a healthcare professional to identify a fasting strategy tailored to their unique biology to monitor for and mitigate negative effects.
Notable Limitations of the Current Research
While fasting is being discussed more and more for a wide range of benefits, the available research is still in its infancy and (like with all research) has its limitations. As has been duly noted by multiple reviews on the effects of fasting on human health2,3,11,12,83,108, there are some notable gaps in the experimental evidence that warrant clarification and should thus be taken into consideration if fasting is something you might like to try.
Although the effects of fasting have been intensively studied for years, human studies have been predominantly restricted to healthy adult populations.2,3,11,12,83,108 Recent research on the effects of fasting on Crohn’s disease and ulcerative colitis have thus far been limited to mostly in vivo animal models of experimental colitis and in vitro tissue culture studies, so their application to humans is inherently limited. The few human studies that have been performed to date have not only yielded mixed results, but also were investigated in small groups of study participants, were conducted for a relatively short duration, and have provided very limited evidence on the impact of fasting in patients with CD.2,3,11,12,83,85,86,108 A clinical trial (NCT04271748) that launched in 2021 is currently investigating the effect of TRF on the gut microbiome and clinical outcomes in patients with Crohn’s.109,110 Even so, the IBD Centre of British Columbia has concluded that there is not yet enough evidence to advocate the therapeutic use of fasting for IBDers.85
The Fasting Trifecta
We’ve discussed the various types of fasting and some potential benefits and risks, but how exactly might one incorporate fasting into their life? There are three critical variables to consider before and throughout your fasting experience: type and frequency of fasting (how and how often you fast), time window of fasting (when you fast), and duration of fasting (how long you fast for).
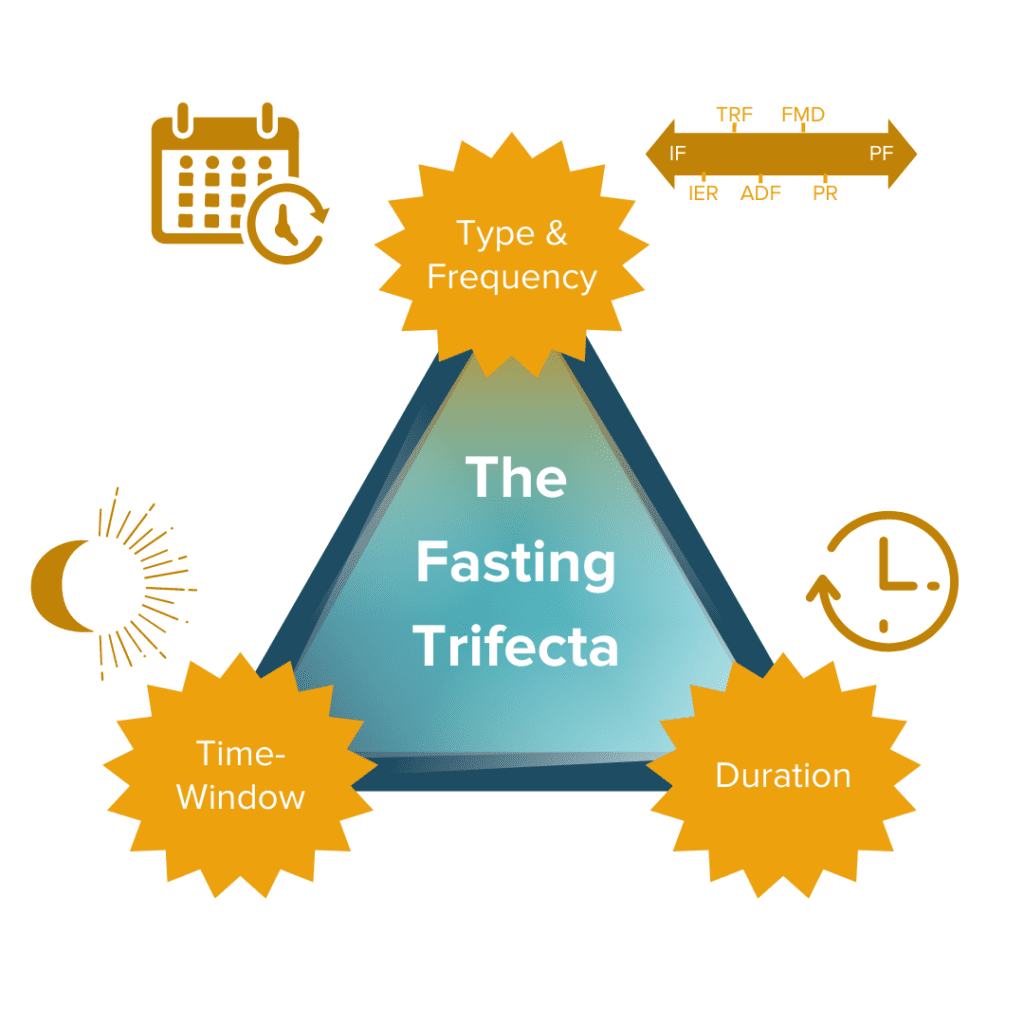
How well a fasting regimen (if any) will work for each individual depends on the type of fasting and the ‘fasting trifecta’ variations and combinations we choose (or don’t choose) to pursue. Our specific biologies, environments, lifestyles, other behavioral characteristics, and countless other factors all come together to determine the best combination of the trifecta that may work the best for each of us individually.
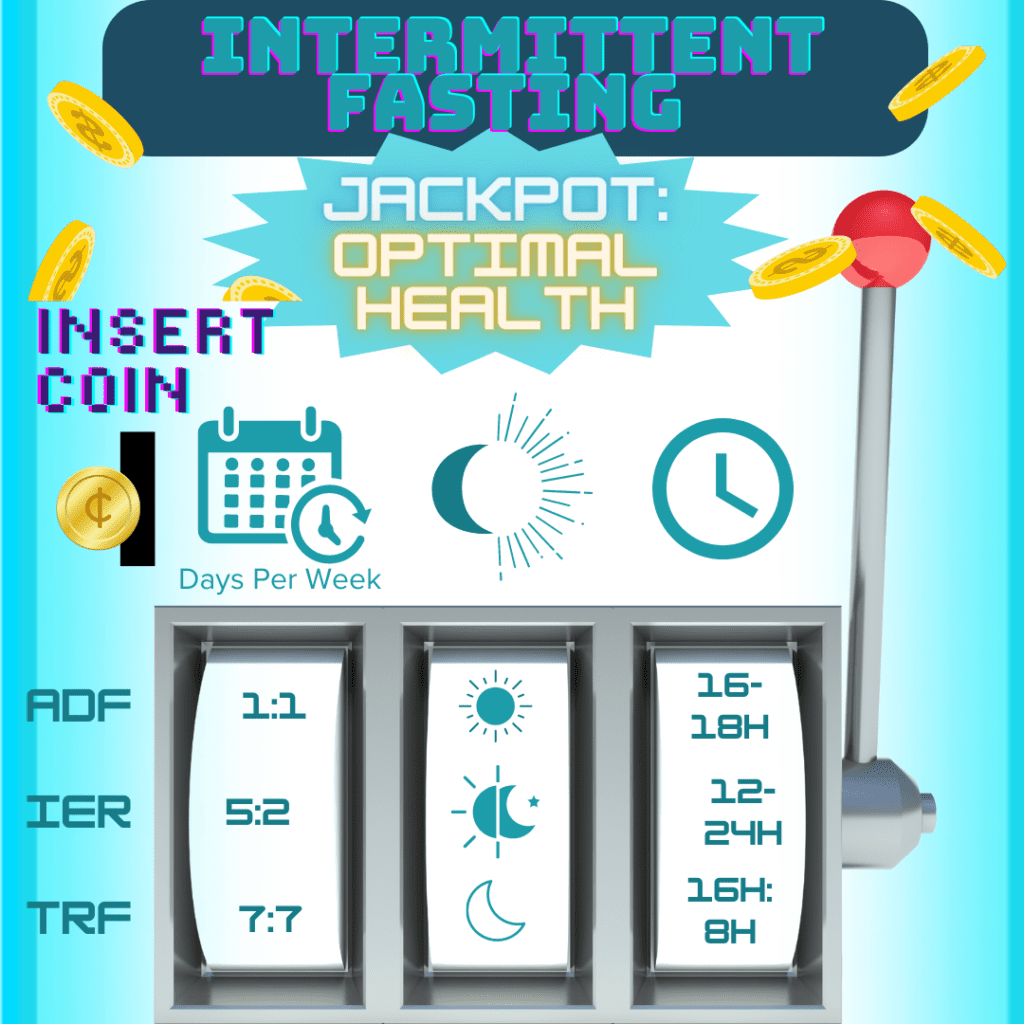
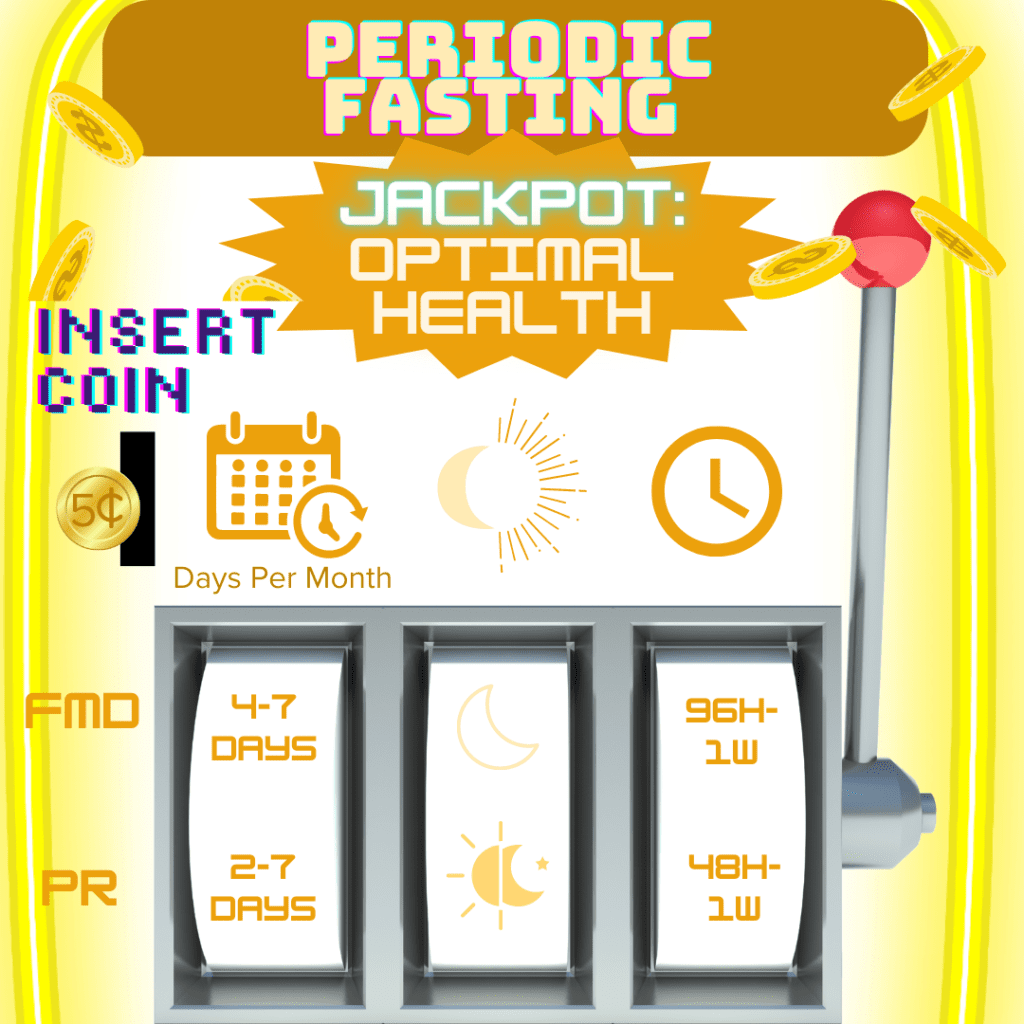
Fasting Type & Frequency (How and how often you choose to fast)
How You Choose To Fast: There are a variety of fasting regimens to choose from, starting with the decision between IF or PF. PF (especially prolonged fasting) is not generally recommended for individuals with CD due to the high risk of malnourishment and related complications.2,3,12,85–88 Although specific variations of PF (like FMD) may have some benefit for the IBD community, there is not enough evidence yet to conclusively advocate its therapeutic use for IBD.85
From there, you can choose which variation of the two (i.e., TRF, ADF, or IER; and FMD or PR) will work best for you! You can use the table from the above section (‘Common Types of Fasting’) as a starting point to explore which ‘how’ might be able to meet your needs.
How Often You Choose To Fast: Frequency of fasting refers to the number of times the fasting regimen is repeated within a designated interval or fasting cycle. Interestingly, the refeeding period associated with fasting appears to enhance its regenerative capacity compared to strict CR.3,75(p1),111–113 For each of the five mentioned fasting regimens, examples of their frequencies include:
Fasting Time-Window (When you choose to fast)
Research has shown that time of day heavily influences the impact of fasting. Studies suggest fasting during the evening rather than the day may sync metabolic patterns with circadian biology by aligning food intake with optimal metabolic hormone responses.12
Fasting Duration (How long you choose to fast for)
The type of fasting regimen pursued (i.e., IF vs. PF) will primarily determine the duration of fasting. Duration of fasting refers to the length of time spent in fasting periods (rather than non-fasting) within a given fasting cycle. A recent review3 highlighted the critical role that much shorter (rather than continuous) fasting intervals play in mitigating the adverse side effects associated with chronic DR strategies. Evidence suggests that approximately 12-hour fasting or TRF intervals may offer the most beneficial health effects due to the apparent absence of known harmful effects.3
However, certain fasting intervals can work better or worse for your specific biology within the variations of each regimen (i.e., TRF, ADF, and IER; PR, and FMD).
Examples of Trifecta Combinations from the Scientific Research:
IF for 12-48 hours done every 1-7 days and PF for 2-7 days done once or less per month may harness the capacity to ward off and improve disease activity;3 however, research definitively identifying its impact on cellular aging processes, the mechanisms at play, and how these mechanisms interact with the primary mechanisms active in IBD pathology is still in its infancy.3 PF can be repeated but should not be done frequently (less than once every two weeks, >1x/2 weeks).3
Personalizing Your Combination of the Fasting Trifecta to ‘Hit the Jackpot’
Now that we’ve reviewed some common fasting types and potential benefits and risks, you can use this information to help determine which combination of the fasting trifecta might work best for you!
To help us understand the relationship between each of these variables within the context of fasting, let’s consider the simple analogy of winning on a slot machine:
Generally, we can think of fasting as the slot machine, with each variable as its own wheel on the machine. The goal of the slot machine is to hit the jackpot; with fasting, we can broadly define the ‘goal’ as achieving optimal health benefits for an individual’s unique biology. All three wheels must align just right to hit the jackpot with the best combination of fasting frequency, window, and duration!
Hitting the ‘Jackpot’ of Fasting Outcomes: Different combinations of the fasting trifecta’s variables will work differently for each person’s biology. The first choice is deciding which ‘slot machine’ to play (the equivalent of choosing which type of fasting to explore). Then we can ‘play to win’ by pulling the lever, which generates a fasting trifecta combination (the equivalent of exploring one combination of the fasting trifecta’s variables). Whether or not this combination is your ‘jackpot’ will be partly determined by which fasting category and associated regimen you chose to pursue, but also by your own unique contributing factors (such as your biology and lifestyle).
Key Takeaways
Research suggests that significant fasting benefits could include improved overall metabolic health, longevity, and healthspan. However, the use of fasting regimens—particularly the more prolonged and severe variations—also carry unique risks for IBDers (especially those with Crohn’s disease), including weight loss, malnourishment, and worsening of current micronutrient deficiencies. Although some promising research has been accrued to support the benefits of fasting in healthy adult individuals, there is still not enough evidence in humans to definitively advocate its therapeutic use for IBDers. How well a fasting regimen (if any) will work for any one person depends on not only the particular combination of the “fasting trifecta’s” variables, but also on how that combination interacts with their specific biology, environment, lifestyle, other behavioral characteristics, comorbid conditions, and countless other factors. This can make the pursuit of fasting feel overwhelming, but ultimately it means there is room for choice and customization.
All forms of fasting should only be pursued after consulting with and notifying your healthcare team. If you and your healthcare team think that fasting is something you’d like to explore, consider how the “fasting trifecta’s” variables—type and frequency of fasting, fasting time window, and fasting duration—might work best for your medical and lifestyle needs.
Are you interested in learning how to leverage your own unique biology to craft a rounded IBD remission protocol that’s tailored specifically to meet your unique needs and goals? IBDCoach has developed a comprehensive Remission Master Plan to help empower IBDers to use their knowledge to fight back against IBD. Enroll in our Free Online Microcourse: The Foundations of Remission, or Schedule an Admissions Call to learn how to get started today!
1. THESE ARE THE MOST POPULAR DIET TRENDS ON TIKTOK IN 2022. YorkTest US. Published February 23, 2022. Accessed October 3, 2022. https://www.yorktest.com/us/blog/most-popular-diet-trends-on-tiktok-in-2022/
2. Longo VD, Mattson MP. Fasting: Molecular Mechanisms and Clinical Applications. Cell Metab. 2014;19(2):181-192. doi:10.1016/j.cmet.2013.12.008
3. Longo VD, Di Tano M, Mattson MP, Guidi N. Intermittent and periodic fasting, longevity and disease. Nat Aging. 2021;1(1):47-59. doi:10.1038/s43587-020-00013-3
4. Abe T, Nakajima A, Satoh N, Ohkoshi M, Sakuragi S, Koizumi A. Suppression of Experimental Autoimmune Uveoretinitis by Dietary Calorie Restriction. Jpn J Ophthalmol. 2001;45(1):46-52. doi:10.1016/S0021-5155(00)00303-8
5. Jolly CA, Fernandes G. Diet Modulates Th-1 and Th-2 Cytokine Production in the Peripheral Blood of Lupus-Prone Mice. J Clin Immunol. 1999;19(3):172-178. doi:10.1023/A:1020503727157
6. Fontana L, Klein S. Aging, Adiposity, and Calorie Restriction. JAMA. 2007;297(9):986-994. doi:10.1001/jama.297.9.986
7. Extending Healthy Life Span—From Yeast to Humans | Science. Accessed October 18, 2022. https://www.science.org/doi/abs/10.1126/science.1172539
8. Kristan DM. Chronic calorie restriction increases susceptibility of laboratory mice (Mus musculus) to a primary intestinal parasite infection. Aging Cell. 2007;6(6):817-825. doi:10.1111/j.1474-9726.2007.00345.x
9. Gardner EM. Caloric Restriction Decreases Survival of Aged Mice in Response to Primary Influenza Infection. J Gerontol Ser A. 2005;60(6):688-694. doi:10.1093/gerona/60.6.688
10. Zhang X, Yang S, Chen J, Su Z. Unraveling the Regulation of Hepatic Gluconeogenesis. Front Endocrinol. 2019;9:802. doi:10.3389/fendo.2018.00802
11. Sanvictores T, Casale J, Huecker MR. Physiology, Fasting. StatPearls Publishing; 2021. Accessed October 4, 2022. https://www.ncbi.nlm.nih.gov/books/NBK534877/
12. Patterson RE, Sears DD. Metabolic Effects of Intermittent Fasting. Annu Rev Nutr. 2017;37(1):371-393. doi:10.1146/annurev-nutr-071816-064634
13. Rangan P, Choi I, Wei M, et al. Fasting-Mimicking Diet Modulates Microbiota and Promotes Intestinal Regeneration to Reduce Inflammatory Bowel Disease Pathology. Cell Rep. 2019;26(10):2704-2719.e6. doi:10.1016/j.celrep.2019.02.019
14. Wei M, Brandhorst S, Shelehchi M, et al. Fasting-mimicking diet and markers/risk factors for aging, diabetes, cancer, and cardiovascular disease. Sci Transl Med. 2017;9(377):eaai8700. doi:10.1126/scitranslmed.aai8700
15. Razavi R, Parvaresh A, Abbasi B, et al. The alternate-day fasting diet is a more effective approach than a calorie restriction diet on weight loss and hs-CRP levels. Int J Vitam Nutr Res Int Z Vitam- Ernahrungsforschung J Int Vitaminol Nutr. 2021;91(3-4):242-250. doi:10.1024/0300-9831/a000623
16. Kord Varkaneh H, Salehi sahlabadi A, Găman MA, et al. Effects of the 5:2 intermittent fasting diet on non-alcoholic fatty liver disease: A randomized controlled trial. Front Nutr. 2022;9. Accessed October 6, 2022. https://www.frontiersin.org/articles/10.3389/fnut.2022.948655
17. McAllister MJ, Pigg BL, Renteria LI, Waldman HS. Time-restricted feeding improves markers of cardiometabolic health in physically active college-age men: a 4-week randomized pre-post pilot study. Nutr Res N Y N. 2020;75:32-43. doi:10.1016/j.nutres.2019.12.001
18. Jordan S, Tung N, Casanova-Acebes M, et al. Dietary Intake Regulates the Circulating Inflammatory Monocyte Pool. Cell. 2019;178(5):1102-1114.e17. doi:10.1016/j.cell.2019.07.050
19. Changes in C-Reactive Protein from Low-Fat Diet and/or Physical Activity in Men and Women With and Without Metabolic Syndrome – PMC. Accessed October 6, 2022. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2789861/
20. Su J, Braat H, Peppelenbosch MP. Gut Microbiota-Derived Propionate Production May Explain Beneficial Effects of Intermittent Fasting in Experimental Colitis. J Crohns Colitis. 2021;15(6):1081-1082. doi:10.1093/ecco-jcc/jjaa248
21. Bajic D, Niemann A, Hillmer AK, et al. Gut Microbiota-Derived Propionate Regulates the Expression of Reg3 Mucosal Lectins and Ameliorates Experimental Colitis in Mice. J Crohns Colitis. 2020;14(10):1462-1472. doi:10.1093/ecco-jcc/jjaa065
22. Rinninella E, Cintoni M, Raoul P, et al. Gut Microbiota during Dietary Restrictions: New Insights in Non-Communicable Diseases. Microorganisms. 2020;8(8):1140. doi:10.3390/microorganisms8081140
23. Beli E, Yan Y, Moldovan L, et al. Restructuring of the Gut Microbiome by Intermittent Fasting Prevents Retinopathy and Prolongs Survival in db/db Mice. Diabetes. 2018;67(9):1867-1879. doi:10.2337/db18-0158
24. Liu Z, Dai X, Zhang H, et al. Gut microbiota mediates intermittent-fasting alleviation of diabetes-induced cognitive impairment. Nat Commun. 2020;11(1):855. doi:10.1038/s41467-020-14676-4
25. Akutko K, Stawarski A. Probiotics, Prebiotics and Synbiotics in Inflammatory Bowel Diseases. J Clin Med. 2021;10(11):2466. doi:10.3390/jcm10112466
26. Rinninella E, Raoul P, Cintoni M, et al. What is the Healthy Gut Microbiota Composition? A Changing Ecosystem across Age, Environment, Diet, and Diseases. Microorganisms. 2019;7(1):14. doi:10.3390/microorganisms7010014
27. Repoila F, Le Bohec F, Guérin C, et al. Adaptation of the gut pathobiont Enterococcus faecalis to deoxycholate and taurocholate bile acids. Sci Rep. 2022;12(1):8485. doi:10.1038/s41598-022-12552-3
28. Sayols-Baixeras S, Dekkers KF, Hammar U, et al. Streptococcus species abundance in the gut is linked to subclinical coronary atherosclerosis in 8,973 participants from the SCAPIS cohort. Published online May 25, 2022:2022.05.25.22275561. doi:10.1101/2022.05.25.22275561
29. Tan J, Zhong Z, Tang Y, Qin W. Intestinal dysbiosis featuring abundance of Streptococcus associates with Henoch-Schönlein purpura nephritis (IgA vasculitis with nephritis) in adult. BMC Nephrol. 2022;23(1):10. doi:10.1186/s12882-021-02638-x
30. Cureus | Small Intestinal Bacterial Overgrowth: Comprehensive Review of Diagnosis, Prevention, and Treatment Methods. Accessed October 28, 2022. https://www.cureus.com/articles/32682-small-intestinal-bacterial-overgrowth-comprehensive-review-of-diagnosis-prevention-and-treatment-methods
31. Zou Z, Tao T, Li H, Zhu X. mTOR signaling pathway and mTOR inhibitors in cancer: progress and challenges. Cell Biosci. 2020;10(1):31. doi:10.1186/s13578-020-00396-1
32. Eaden JA, Abrams KR, Mayberry JF. The risk of colorectal cancer in ulcerative colitis: a meta-analysis. Gut. 2001;48(4):526-535. doi:10.1136/gut.48.4.526
33. Jess T, Rungoe C, Peyrin–Biroulet L. Risk of Colorectal Cancer in Patients With Ulcerative Colitis: A Meta-analysis of Population-Based Cohort Studies. Clin Gastroenterol Hepatol. 2012;10(6):639-645. doi:10.1016/j.cgh.2012.01.010
34. Lutgens MWMD, Vleggaar FP, Schipper MEI, et al. High frequency of early colorectal cancer in inflammatory bowel disease. Gut. 2008;57(9):1246-1251. doi:10.1136/gut.2007.143453
35. Selinger CP, Andrews JM, Titman A, et al. Long-term Follow-up Reveals Low Incidence of Colorectal Cancer, but Frequent Need for Resection, Among Australian Patients With Inflammatory Bowel Disease. Clin Gastroenterol Hepatol. 2014;12(4):644-650. doi:10.1016/j.cgh.2013.05.017
36. Colorectal Cancer Risk in IBD. Crohn’s & Colitis Foundation. Accessed October 27, 2022. https://www.crohnscolitisfoundation.org/science-and-professionals/education-resources/colorectal-cancer-risk-ibd
37. Lee C, Raffaghello L, Brandhorst S, et al. Fasting Cycles Retard Growth of Tumors and Sensitize a Range of Cancer Cell Types to Chemotherapy. Sci Transl Med. 2012;4(124):124ra27. doi:10.1126/scitranslmed.3003293
38. Guevara-Aguirre J, Balasubramanian P, Guevara-Aguirre M, et al. Growth Hormone Receptor Deficiency Is Associated with a Major Reduction in Pro-Aging Signaling, Cancer, and Diabetes in Humans. Sci Transl Med. 2011;3(70):70ra13-70ra13. doi:10.1126/scitranslmed.3001845
39. Lee C, Safdie FM, Raffaghello L, et al. Reduced Levels of IGF-I Mediate Differential Protection of Normal and Cancer Cells in Response to Fasting and Improve Chemotherapeutic Index. Cancer Res. 2010;70(4):1564-1572. doi:10.1158/0008-5472.CAN-09-3228
40. Descamps O, Riondel J, Ducros V, Roussel AM. Mitochondrial production of reactive oxygen species and incidence of age-associated lymphoma in OF1 mice: Effect of alternate-day fasting. Mech Ageing Dev. 2005;126(11):1185-1191. doi:10.1016/j.mad.2005.06.007
41. Berrigan D, Perkins SN, Haines DC, Hursting SD. Adult-onset calorie restriction and fasting delay spontaneous tumorigenesis in p53-deficient mice. Carcinogenesis. 2002;23(5):817-822. doi:10.1093/carcin/23.5.817
42. Shi Y, Felley-Bosco E, Marti TM, Orlowski K, Pruschy M, Stahel RA. Starvation-induced activation of ATM/Chk2/p53 signaling sensitizes cancer cells to cisplatin. BMC Cancer. 2012;12(1):571. doi:10.1186/1471-2407-12-571
43. Raffaghello L, Lee C, Safdie FM, et al. Starvation-dependent differential stress resistance protects normal but not cancer cells against high-dose chemotherapy. Proc Natl Acad Sci. 2008;105(24):8215-8220. doi:10.1073/pnas.0708100105
44. Ahmet I, Wan R, Mattson MP, Lakatta EG, Talan M. Cardioprotection by Intermittent Fasting in Rats. Circulation. 2005;112(20):3115-3121. doi:10.1161/CIRCULATIONAHA.105.563817
45. Pedersen CR, Hagemann I, Bock T, Buschard K. Intermittent Feeding and Fasting Reduces Diabetes Incidence in BB Rats. Autoimmunity. 1999;30(4):243-250. doi:10.3109/08916939908993805
46. Arumugam TV, Phillips TM, Cheng A, Morrell CH, Mattson MP, Wan R. Age and energy intake interact to modify cell stress pathways and stroke outcome. Ann Neurol. 2010;67(1):41-52. doi:10.1002/ana.21798
47. Bruce-Keller AJ, Umberger G, McFall R, Mattson MP. Food restriction reduces brain damage and improves behavioral outcome following excitotoxic and metabolic insults. Ann Neurol. 1999;45(1):8-15. doi:10.1002/1531-8249(199901)45:1<8::AID-ART4>3.0.CO;2-V
48. Halagappa VKM, Guo Z, Pearson M, et al. Intermittent fasting and caloric restriction ameliorate age-related behavioral deficits in the triple-transgenic mouse model of Alzheimer’s disease. Neurobiol Dis. 2007;26(1):212-220. doi:10.1016/j.nbd.2006.12.019
49. Duan W, Guo Z, Jiang H, Ware M, Li XJ, Mattson MP. Dietary restriction normalizes glucose metabolism and BDNF levels, slows disease progression, and increases survival in huntingtin mutant mice. Proc Natl Acad Sci. 2003;100(5):2911-2916. doi:10.1073/pnas.0536856100
50. Duan W, Mattson MP. Dietary restriction and 2-deoxyglucose administration improve behavioral outcome and reduce degeneration of dopaminergic neurons in models of Parkinson’s disease. J Neurosci Res. 1999;57(2):195-206. doi:10.1002/(SICI)1097-4547(19990715)57:2<195::AID-JNR5>3.0.CO;2-P
51. Griffioen KJ, Wan R, Brown TR, et al. Aberrant heart rate and brainstem brain-derived neurotrophic factor (BDNF) signaling in a mouse model of Huntington’s disease. Neurobiol Aging. 2012;33(7):1481.e1-1481.e5. doi:10.1016/j.neurobiolaging.2011.11.030
52. Alirezaei M, Kemball CC, Flynn CT, Wood MR, Whitton JL, Kiosses WB. Short-term fasting induces profound neuronal autophagy. Autophagy. 2010;6(6):702-710. doi:10.4161/auto.6.6.12376
53. Martinez-Lopez N, Tarabra E, Toledo M, et al. System-wide Benefits of Intermeal Fasting by Autophagy. Cell Metab. 2017;26(6):856-871.e5. doi:10.1016/j.cmet.2017.09.020
54. Bagherniya M, Butler AE, Barreto GE, Sahebkar A. The effect of fasting or calorie restriction on autophagy induction: A review of the literature. Ageing Res Rev. 2018;47:183-197. doi:10.1016/j.arr.2018.08.004
55. Moore MN, Shaw JP, Ferrar Adams DR, Viarengo A. Anti-oxidative cellular protection effect of fasting-induced autophagy as a mechanism for hormesis. Mar Environ Res. 2015;107:35-44. doi:10.1016/j.marenvres.2015.04.001
56. Phillips MCL. Fasting as a Therapy in Neurological Disease. Nutrients. 2019;11(10):2501. doi:10.3390/nu11102501
57. Stockman MC, Thomas D, Burke J, Apovian CM. Intermittent Fasting: Is the Wait Worth the Weight? Curr Obes Rep. 2018;7(2):172-185. doi:10.1007/s13679-018-0308-9
58. Hara T, Nakamura K, Matsui M, et al. Suppression of basal autophagy in neural cells causes neurodegenerative disease in mice. Nature. 2006;441(7095):885-889. doi:10.1038/nature04724
59. Komatsu M, Waguri S, Chiba T, et al. Loss of autophagy in the central nervous system causes neurodegeneration in mice. Nature. 2006;441(7095):880-884. doi:10.1038/nature04723
60. Alirezaei M, Kiosses WB, Flynn CT, Brady NR, Fox HS. Disruption of Neuronal Autophagy by Infected Microglia Results in Neurodegeneration. PLOS ONE. 2008;3(8):e2906. doi:10.1371/journal.pone.0002906
61. Orvedahl A, Levine B. Eating the enemy within: autophagy in infectious diseases. Cell Death Differ. 2009;16(1):57-69. doi:10.1038/cdd.2008.130
62. Mizushima N, Levine B, Cuervo AM, Klionsky DJ. Autophagy fights disease through cellular self-digestion. Nature. 2008;451(7182):1069-1075. doi:10.1038/nature06639
63. Rioux JD, Xavier RJ, Taylor KD, et al. Genome-wide association study identifies new susceptibility loci for Crohn disease and implicates autophagy in disease pathogenesis. Nat Genet. 2007;39(5):596-604. doi:10.1038/ng2032
64. McCarroll SA, Huett A, Kuballa P, et al. Deletion polymorphism upstream of IRGM associated with altered IRGM expression and Crohn’s disease. Nat Genet. 2008;40(9):1107-1112. doi:10.1038/ng.215
65. Yin H, Wu H, Chen Y, et al. The Therapeutic and Pathogenic Role of Autophagy in Autoimmune Diseases. Front Immunol. 2018;9. Accessed October 6, 2022. https://www.frontiersin.org/articles/10.3389/fimmu.2018.01512
66. What is Health Span? Accessed October 5, 2022. https://www.merriam-webster.com/words-at-play/what-is-health-span
67. Varady KA, Cienfuegos S, Ezpeleta M, Gabel K. Cardiometabolic Benefits of Intermittent Fasting. Annu Rev Nutr. 2021;41(1):333-361. doi:10.1146/annurev-nutr-052020-041327
68. Sundqvist T, Lindström F, Magnusson KE, Sköldstam L, Stjernström I, Tagesson C. Influence of fasting on intestinal permeability and disease activity in patients with rheumatoid arthritis. Scand J Rheumatol. 1982;11(1):33-38. doi:10.3109/03009748209098111
69. Jamshed H, Beyl RA, Della Manna DL, Yang ES, Ravussin E, Peterson CM. Early Time-Restricted Feeding Improves 24-Hour Glucose Levels and Affects Markers of the Circadian Clock, Aging, and Autophagy in Humans. Nutrients. 2019;11(6):1234. doi:10.3390/nu11061234
70. Attinà A, Leggeri C, Paroni R, et al. Fasting: How to Guide. Nutrients. 2021;13(5):1570. doi:10.3390/nu13051570
71. Anson RM, Guo Z, de Cabo R, et al. Intermittent fasting dissociates beneficial effects of dietary restriction on glucose metabolism and neuronal resistance to injury from calorie intake. Proc Natl Acad Sci. 2003;100(10):6216-6220. doi:10.1073/pnas.1035720100
72. Mager DE, Wan R, Brown M, et al. Caloric restriction and intermittent fasting alter spectral measures of heart rate and blood pressure variability in rats. FASEB J. 2006;20(6):631-637. doi:10.1096/fj.05-5263com
73. Wan R, Camandola S, Mattson MP. Intermittent fasting and dietary supplementation with 2-deoxy-D-glucose improve functional and metabolic cardiovascular risk factors in rats. FASEB J Off Publ Fed Am Soc Exp Biol. 2003;17(9):1133-1134. doi:10.1096/fj.02-0996fje
74. Wilkinson MJ, Manoogian ENC, Zadourian A, et al. Ten-Hour Time-Restricted Eating Reduces Weight, Blood Pressure, and Atherogenic Lipids in Patients with Metabolic Syndrome. Cell Metab. 2020;31(1):92-104.e5. doi:10.1016/j.cmet.2019.11.004
75. Cheng CW, Adams GB, Perin L, et al. Prolonged Fasting Reduces IGF-1/PKA to Promote Hematopoietic-Stem-Cell-Based Regeneration and Reverse Immunosuppression. Cell Stem Cell. 2014;14(6):810-823. doi:10.1016/j.stem.2014.04.014
76. Aguirre GA, De Ita JR, de la Garza RG, Castilla-Cortazar I. Insulin-like growth factor-1 deficiency and metabolic syndrome. J Transl Med. 2016;14:3. doi:10.1186/s12967-015-0762-z
77. Adawi M, Watad A, Brown S, et al. Ramadan Fasting Exerts Immunomodulatory Effects: Insights from a Systematic Review. Front Immunol. 2017;8. Accessed September 27, 2022. https://www.frontiersin.org/articles/10.3389/fimmu.2017.01144
78. Michielan A, D’Incà R. Intestinal Permeability in Inflammatory Bowel Disease: Pathogenesis, Clinical Evaluation, and Therapy of Leaky Gut. Mediators Inflamm. 2015;2015:628157. doi:10.1155/2015/628157
79. Chen T, Kim CY, Kaur A, et al. Dietary fibre-based SCFA mixtures promote both protection and repair of intestinal epithelial barrier function in a Caco-2 cell model. Food Funct. 2017;8(3):1166-1173.
80. Thursby E, Juge N. Introduction to the human gut microbiota. Biochem J. 2017;474(11):1823-1836. doi:10.1042/BCJ20160510
81. Martin-Gallausiaux C, Marinelli L, Blottière HM, Larraufie P, Lapaque N. SCFA: mechanisms and functional importance in the gut. Proc Nutr Soc. 2021;80(1):37-49. doi:10.1017/S0029665120006916
82. Ige S, Adebola I, Adio O, Ojoye O. The Therapeutic Potential of Time-Restricted Fasting on Experimental Ulcerative Colitis. J Adv Med Pharm Sci. Published online November 6, 2020:25-33. doi:10.9734/jamps/2020/v22i830188
83. Celiberto LS, Graef FA, Healey GR, et al. Inflammatory bowel disease and immunonutrition: novel therapeutic approaches through modulation of diet and the gut microbiome. Immunology. 2018;155(1):36-52. doi:10.1111/imm.12939
84. Parada Venegas D, De la Fuente MK, Landskron G, et al. Short Chain Fatty Acids (SCFAs)-Mediated Gut Epithelial and Immune Regulation and Its Relevance for Inflammatory Bowel Diseases. Front Immunol. 2019;10:277. doi:10.3389/fimmu.2019.00277
85. Cruz E. Can Intermittent Fasting Help with Inflammatory Bowel Disease? The IBD Centre of BC. Published July 28, 2021. Accessed September 27, 2022. https://www.ibdcentrebc.ca/2021/07/intermittent-fasting-on-ibd/
86. Should You Try Intermittent Fasting if You Have Crohn’s? EverydayHealth.com. Accessed September 27, 2022. https://www.everydayhealth.com/crohns-disease/should-you-try-intermittent-fasting-if-you-have-crohns/
87. Goldhamer A, Lisle D, Parpia B, Anderson SV, Campbell TC. Medically supervised water-only fasting in the treatment of hypertension. J Manipulative Physiol Ther. 2001;24(5):335-339. doi:10.1067/mmt.2001.115263
88. Goldhamer AC, Lisle DJ, Sultana P, et al. Medically Supervised Water-Only Fasting in the Treatment of Borderline Hypertension. J Altern Complement Med. 2002;8(5):643-650. doi:10.1089/107555302320825165
89. Crohn’s and Colitis Foundation. Managing Flares and IBD Symptoms. Published online July 2019. Accessed November 1, 2022. https://www.crohnscolitisfoundation.org/sites/default/files/2019-07/managing-flares-brochure-final-online.pdf
90. What is a Flare? Crohn’s & Colitis Foundation. Accessed November 1, 2022. https://www.crohnscolitisfoundation.org/ibd-me/what-is-a-flare-comic
91. Malnutrition and IBD. Crohn’s & Colitis Foundation. Accessed October 17, 2022. https://www.crohnscolitisfoundation.org/diet-and-nutrition/malnutrition-and-ibd
92. Signs and Symptoms of Crohn’s Disease. Crohn’s & Colitis Foundation. Accessed April 4, 2022. https://www.crohnscolitisfoundation.org/what-is-crohns-disease/symptoms
93. Signs and Symptoms of Ulcerative Colitis. Crohn’s & Colitis Foundation. Accessed November 1, 2022. https://www.crohnscolitisfoundation.org/what-is-ulcerative-colitis/symptoms
94. Pancreas Hormones. Endocrine Society. Accessed October 5, 2022. https://www.endocrine.org/patient-engagement/endocrine-library/hormones-and-endocrine-function/pancreas-hormones
95. Zatorski H, Salaga M, Zielińska M, et al. Colonic inflammation induces changes in glucose levels through modulation of incretin system. Pharmacol Rep. 2021;73(6):1670-1679. doi:10.1007/s43440-021-00327-y
96. Wicker C, Roda C, Perry A, et al. Infectious and digestive complications in glycogen storage disease type Ib: Study of a French cohort. Mol Genet Metab Rep. 2020;23:100581. doi:10.1016/j.ymgmr.2020.100581
97. Kang EA, Han K, Chun J, et al. Increased Risk of Diabetes in Inflammatory Bowel Disease Patients: A Nationwide Population-Based Study in Korea. J Clin Med. 2019;8(3):343. doi:10.3390/jcm8030343
98. Mosek A, Korczyn AD. Fasting Headache, Weight Loss, and Dehydration. Headache J Head Face Pain. 1999;39(3):225-227. doi:10.1046/j.1526-4610.1999.3903225.x
99. Torelli P, Evangelista A, Bini A, Castellini P, Lambru G, Manzoni GC. Fasting Headache: A Review of the Literature and New Hypotheses. Headache J Head Face Pain. 2009;49(5):744-752. doi:10.1111/j.1526-4610.2009.01390.x
100. Drescher MJ, Elstein Y. Prophylactic COX 2 Inhibitor: An End to the Yom Kippur Headache. Headache J Head Face Pain. 2006;46(10):1487-1491. doi:10.1111/j.1526-4610.2006.00609.x
101. Harvie MN, Pegington M, Mattson MP, et al. The effects of intermittent or continuous energy restriction on weight loss and metabolic disease risk markers: a randomized trial in young overweight women. Int J Obes. 2011;35(5):714-727. doi:10.1038/ijo.2010.171
102. Harvie M, Wright C, Pegington M, et al. The effect of intermittent energy and carbohydrate restriction v. daily energy restriction on weight loss and metabolic disease risk markers in overweight women. Br J Nutr. 2013;110(8):1534-1547. doi:10.1017/S0007114513000792
103. Johnson JB, Summer W, Cutler RG, et al. Alternate day calorie restriction improves clinical findings and reduces markers of oxidative stress and inflammation in overweight adults with moderate asthma. Free Radic Biol Med. 2007;42(5):665-674. doi:10.1016/j.freeradbiomed.2006.12.005
104. Varady KA, Bhutani S, Klempel MC, et al. Alternate day fasting for weight loss in normal weight and overweight subjects: a randomized controlled trial. Nutr J. 2013;12(1):146. doi:10.1186/1475-2891-12-146
105. Harvie M, Howell A. Potential Benefits and Harms of Intermittent Energy Restriction and Intermittent Fasting Amongst Obese, Overweight and Normal Weight Subjects—A Narrative Review of Human and Animal Evidence. Behav Sci. 2017;7(1):4. doi:10.3390/bs7010004
106. Laessle RG, Platte P, Schweiger U, Pirke KM. Biological and psychological correlates of intermittent dieting behavior in young women. A model for bulimia nervosa. Physiol Behav. 1996;60(1):1-5. doi:10.1016/0031-9384(95)02215-5
107. Heilbronn LK, Smith SR, Martin CK, Anton SD, Ravussin E. Alternate-day fasting in nonobese subjects: effects on body weight, body composition, and energy metabolism. Am J Clin Nutr. 2005;81(1):69-73. doi:10.1093/ajcn/81.1.69
108. Nair PMK, Khawale PG. Role of therapeutic fasting in women’s health: An overview. J -Life Health. 2016;7(2):61-64. doi:10.4103/0976-7800.185325
109. University WMC of C. The Impact of Time Restricted Feeding in Crohn’s Disease: Crohn’s Disease (CD) Clinical Trial… TrialBulletin.com. Accessed September 27, 2022. https://trialbulletin.com/lib/entry/ct-04271748
110. Weill Medical College of Cornell University. The Impact of Time Restricted Feeding in Crohn’s Disease. clinicaltrials.gov; 2022. Accessed October 11, 2022. https://clinicaltrials.gov/ct2/show/NCT04271748
111. Brandhorst S, Choi IY, Wei M, et al. A Periodic Diet that Mimics Fasting Promotes Multi-System Regeneration, Enhanced Cognitive Performance, and Healthspan. Cell Metab. 2015;22(1):86-99. doi:10.1016/j.cmet.2015.05.012
112. Choi IY, Piccio L, Childress P, et al. A Diet Mimicking Fasting Promotes Regeneration and Reduces Autoimmunity and Multiple Sclerosis Symptoms. Cell Rep. 2016;15(10):2136-2146. doi:10.1016/j.celrep.2016.05.009
113. Lazare S, Ausema A, Reijne AC, van Dijk G, van Os R, de Haan G. Lifelong dietary intervention does not affect hematopoietic stem cell function. Exp Hematol. 2017;53:26-30. doi:10.1016/j.exphem.2017.06.002