Through its interactions with the microbiome and gut-brain axis, the endocannabinoid system may offer a novel pathway for managing symptoms of Crohn’s disease and ulcerative colitis.
The information below is for educational purposes only and is not a substitute for medical care. Always consult your doctor if you are seeking medical advice, a diagnosis, or treatment for your condition.
Introduction: What is IBD?
Inflammatory bowel diseases (IBDs), such as Crohn’s disease (CD) and ulcerative colitis (UC), are often characterized by symptoms such as abdominal pain, cramps, diarrhea, nausea, and vomiting.1 Although Crohn’s and colitis symptoms can manifest in slightly different ways, both forms of IBD are associated with disease mechanisms involving barrier permeability, inflammation, and changes in microbiome composition. Medical cannabis (Cannabis sativa L.) has recently gained attention for its potential to alleviate symptoms of IBD by acting on the primary biological mechanisms that fuel disease activity. These therapeutic effects of medical marijuana – notably, those implicated in IBD symptom relief – are mediated through the body’s naturally-occurring endocannabinoid system (ECS).1,2 Through its interactions with the microbiome and gut-brain axis (collectively referred to as the microbiota-gut-brain axis, MGBA), the ECS may offer a novel framework for identifying future therapeutic targets for the treatment of IBD and its symptoms.
What is the Endocannabinoid System (ECS)?
The therapeutic benefits of medical cannabis (Cannabis sativa L.) are derived from the exquisitely diverse array of bioactive, non-nutritive compounds naturally synthesized by the plant (referred to as ‘phytochemicals’).3 Renowned among both scientists and consumers alike, the most widely known class of phytochemicals produced by cannabis are phytocannabinoids. This class includes compounds such as Δ9-tetrahydrocannabinol (Δ9-THC) – the psychoactive component of cannabis responsible for the ‘high’ following consumption – and cannabidiol (CBD) – a non-psychoactive component associated with analgesic and anti-inflammatory benefits.4 Phytocannabinoids can elicit physiological and psychoactive effects by regulating brain function through interactions with the receptors of the endogenous cannabinoid system referred to as the endocannabinoid system (ECS).1,2
Click here to read more about cannabis, phytocannabinoids, and IBD.
The ECS is a naturally occurring signaling system dispersed throughout the body that modulates the brain and other physiological functions.2 The principal components of the human ECS include:
- Cannabinoid receptors: signal-receiving proteins such as the cannabinoid receptor 1 (CB1R) and cannabinoid receptor 2 (CB2R)1,2
- Endocannabinoids: endogenous lipid-based transmitter molecules (also known as ligands), such as N-arachidonoylethanolamine (anandamide or AEA) and 2-arachidonoylglycerol (2-AG), that send signals by binding to receptors1,2
- Metabolic enzymes: proteins that that biosynthesize (or produce) and degrade (or break down) AEA and 2-AG1,2
Table 1: Key endocannabinids and the enzymes that synthesize and degrade them.
| Endocannabinoid | Biosynthetic Enzyme1,2 | Degradative Enzyme1,2 |
| N-Arachidonoylethanolamine (Anandamide or AEA) | N-acylphosphatidylethanolamine-hydrolyzing phospholipase D (NAPE-PLD) | fatty acid amide hydrolase (FAAH) |
| 2-Arachidonoylglycerol (2-AG) | diacylglycerol lipase (DAGL)-α | monoacylglycerol lipase (MAGL) |
In addition to being activated by AEA and 2-AG, CB1R and CB2R can also be activated by certain exogenous phytocannabinoids, such as those produced by the Cannabis sativa L. plant. These exogenous phytocannabinoids include Δ9-THC which activates CB1 receptors in the brain resulting in potent psychoactive properties. CBD, another exogenous cannabinoid, does not bind well to CB1R and CB2R and therefore has markedly reduced psychoactive properties compared to Δ9-THC.4–6 Altogether, this complicated system of naturally occurring receptors can have a diverse array of effects on human health and symptoms of IBD.
The Role of the ECS in Human Health & Disease
The ECS predominantly functions to maintain homeostasis, a state of physiological balance, by altering metabolic and behavioral processes within the brain and other parts of the body in response to internal and external stressors. This homeostatic system is critical in regulating exostasis (a biological process that promotes energy storage and accumulation), stress recovery, and social behavior.1
Disruption of proper ECS functioning leads to altered gut endocannabinoid tone, which has been linked to IBD and a variety of other conditions (including anxiety disorders; post-traumatic stress disorder, PTSD; depression; autism; and eating disorders).1 Endocannabinoid tone is a technical way to describe the overarching state of ECS function in the context of its individual components.7 Specifically, it refers to:
- Endocannabinoid (2-AG and AEA) levels7
- Endocannabinoid metabolism (enzymatic biosynthesis and degradation of 2-AG and AEA)7
- Cannabinoid receptor (CB1R and CB2R) density and functional state7
The Role of the ECS in Gastrointestinal (GI) Health
The homeostatic nature of the ECS extends to the gut, where the ECS serves to regulate GI motility, permeability, and inflammation both:
- peripherally ( influences involuntary physiological responses such as vomiting, nausea, and abdominal sensations)1,2, and
- centrally (influences voluntary body functions involved in information processing and mitigating responses to stimuli such as stress).1,2
Interestingly, research has shown that endocannabinoids and phytocannabinoids may wield inverse effects on gut barrier permeability: while 2-AG and AEA promoted inflammation-associated increases in permeability, phytocannabinoids (i.e., Δ9-THC and CBD) restored it.1,8,9 As the scientific and political landscape surrounding medical cannabis availability, usage, and consumption continues to evolve, many researchers have shifted their investigative efforts towards tapping into the therapeutic potential of medical cannabis for the treatment of a variety of diseases, including IBD.
The Relationship Between the Endocannabinoid System, Microbiota-Gut-Brain Axis, and Mechanisms of Inflammatory Bowel Disease
Mechanisms of IBD
To understand how the ECS influences IBD, we must briefly review the positive feedback loop of IBD pathogenesis: dysbiosis of the gut microbiome and increased intestinal permeability fuels a runaway inflammatory response that can further exacerbate dysbiosis and damage to the intestinal barrier.1,10–12,13(pp37-49)
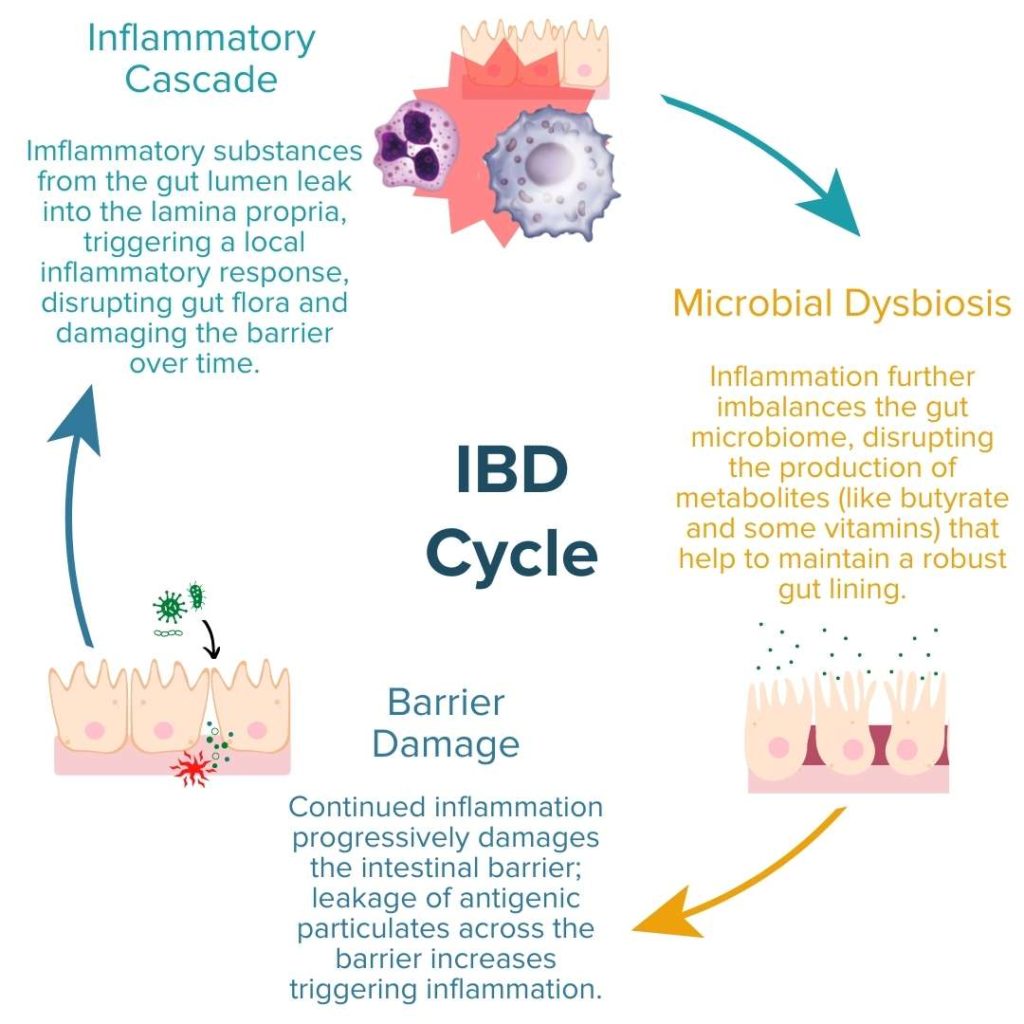
In order to alleviate the symptoms of Crohn’s or UC and heal the gut, well-rounded IBD treatment protocols should seek to address these three primary molecular mechanisms:
- Inflammation (i.e., increased pro-inflammatory response)
- Enteric Microbial Dysbiosis
- Gut Permeability (i.e., increased/impaired permeability of the intestinal lining; ‘leaky’ gut)
Learn More About the Three Mechanisms of IBD Here
All three mechanisms of IBD are influenced by the ECS.1 Notably, gut ECS activity was found to be elevated in mouse models of IBD and in UC patients as a potential way to control inflammation.1,14–18 Further experimental analysis of these results provoked the research teams from these studies to conclude that elevated ECS activity is likely a protective response to colonic inflammation.14,17 Conversely, another study on mice demonstrated that enhanced ECS activity increases gut permeability and bacterial lipopolysaccharide (LPS) plasma levels.19 Increased plasma levels of LPS both indicate increased barrier permeability and cause increased inflammatory response. Although these studies have yielded mixed results, together they highlight the importance of the ECS in intestinal health and its relevance to IBD pathogenesis.
The Gut Microbiome As A Key Mediator Between The Endocannabinoid System And IBD
The microbiota-gut-brain axis (MGBA)1,20 – also known as the gut-brain connection – is a key regulator of the relationship between the ECS and IBD. The MGBA describes the vast, bi-directional signaling network linking the gut and brain. Through this network, the microbial population within the gut (referred to collectively as the ‘enteric microbiota’), the enteric nervous system (ENS), and the central nervous system (CNS) are able to communicate.
Read Our Blog Post About the MGBA and IBD
The four principal routes of communication employed by the MGBA to transmit signals between the gut and brain include the following:1,2,7,11,20–25
- The neurologic pathway connecting the gut to the brain and other elements of the central nervous system
- The immune pathway connecting the gut to functions of the immune system
- The endocrine pathway connecting the gut to the stress response and hormone signals
- The metabolic pathway connecting the gut to other systems of the body through metabolites produced by digestion and the enteric microbiota
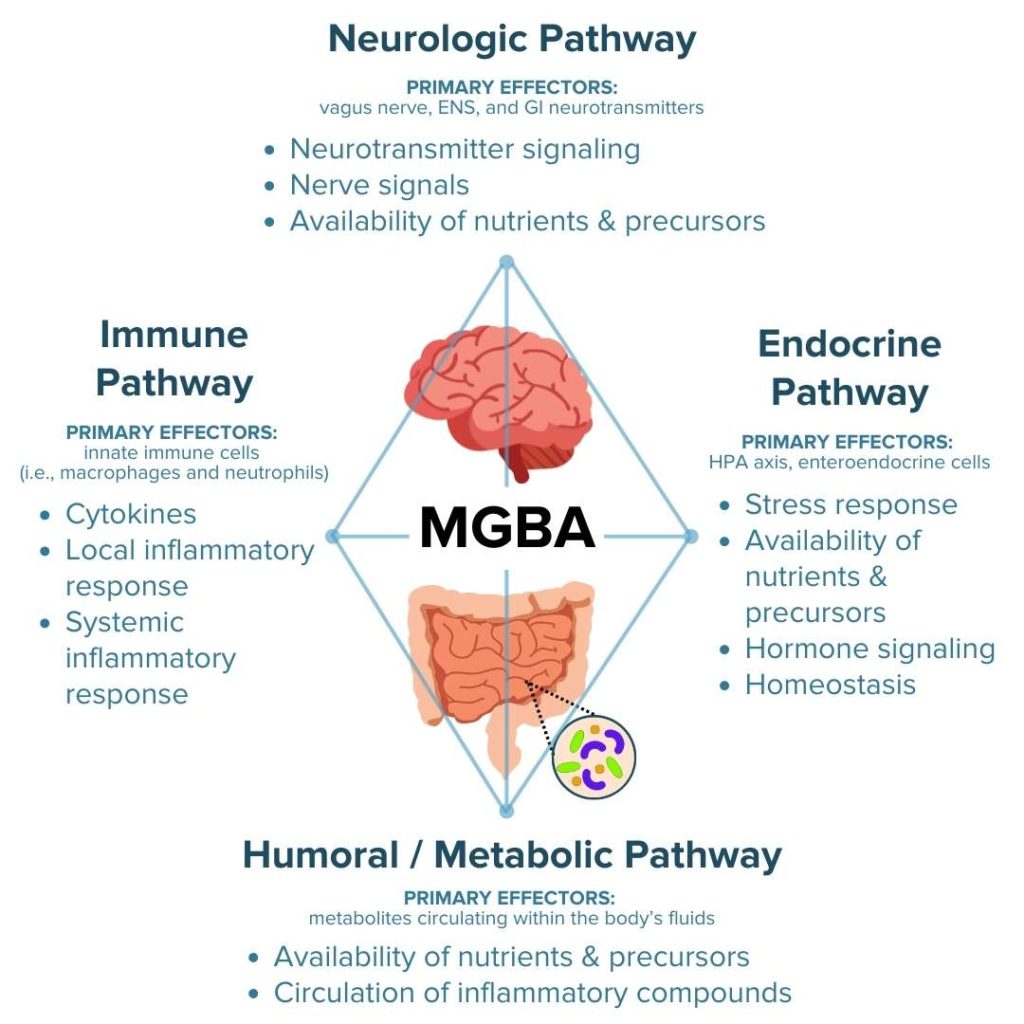
Crosstalk between these MGBA pathways permits the brain to influence intestinal processes involved in regulating local inflammatory activity and gut mechanisms that influence mental state, emotional regulation, neuromuscular function, and regulation of the hypothalamic-pituitary-adrenal (HPA) axis.20
The interplay between the ECS and the four major pathways of the MGBA serves a critical role in regulating stress responses and metabolic processes within the gut.1,2,7 Moreover, a bi-directional communication network between the ECS and enteric microbiota is involved in regulating the gut endocannabinoid tone, gut permeability, gut peptide secretion, and stress resistance within the gut.1,26 The ECS can modulate GI motility, permeability, and inflammation primarily by acting either directly on the brain through the vagus nerve or indirectly via circulation of gut-derived compounds such as neuropeptides (i.e., peptide YY, PYY, and glucose-like peptide 1, GLP-1), gut hormones (i.e., cholecystokinin, CCK), and microbial metabolites (i.e., SCFAs and indole).1
In particular, research has indicated that gut-barrier functions involved in motility, permeability, and nutrient absorption are influenced by the crosstalk between the ECS and MGBA.1 For example, endocannabinoids can restrict gut motility by suppressing activation of excitatory cholinergic neurons, inhibitory vasoactive intestinal peptide (VIP) motor neurons, and calcitonin gene-related peptide (CGRP) neuron-mediated peristaltic reflex through crosstalk with the endocrine and neurologic pathways.1,27 The endocannabinoid AEA regulates the secretion of gut hormones and peptides such as acetylcholine (ACh), CGRP, substance P, and VIP (endocrine pathway) that signal to the brain through afferent vagal nerves throughout the GI tract (neurologic pathway). In the brain, these signals act as neurotransmitters to regulate ascending contraction and descending relaxation (the contractions that constitute peristalsis) to control gut motility. These peristaltic contractions are consequently decreased when the presence of AEA increases in the gut.1,27 Due to the microbiota’s role in regulating endocannabinoid tone, dysbiosis can alter the levels of endocannabinoids in the gut and thereby disrupt ECS-mediated regulation of gut-barrier functions, exacerbate inflammation, and disrupt gut motility and peristalsis.1,28
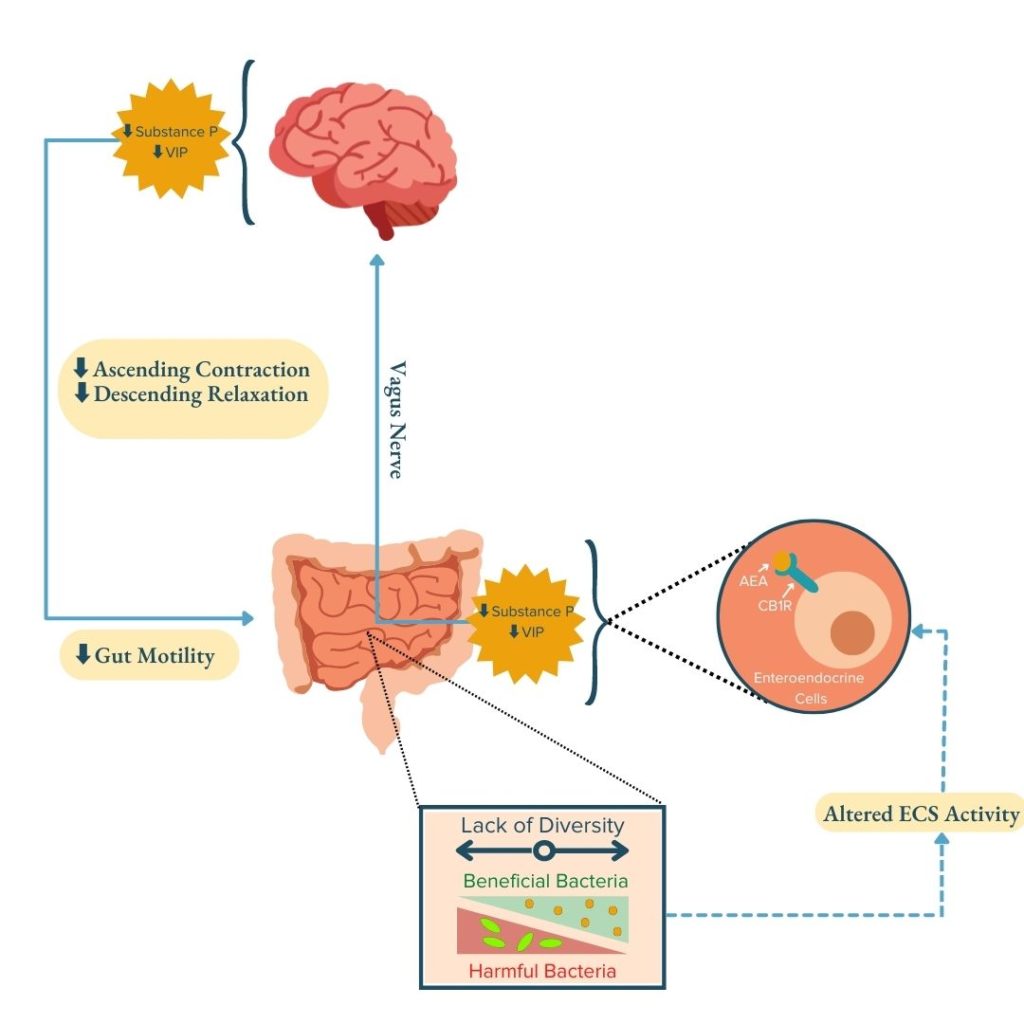
Previous research suggests that susceptibility to ulcerative colitis (UC) and Crohn’s disease could be influenced by genetic variations in the CB1R gene (CNR1). It appears that these variations may alter intestinal inflammation.1,2,14,29–34 CB1R – which is responsible for many of the actions of the ECS – is expressed in afferent vagal nerves throughout the GI tract1,35–37, further highlighting the role of the crosstalk between the ECS and MGBA in regulating GI health.
Altogether, research reveals an interesting relationship between the ECS, the MGBA, and symptoms of IBD. In IBD, an altered cannabinoid tone has been shown to increase gut barrier permeability and increase leakage of bacterial metabolites into circulation or into contact with the vagus nerve, which augments the positive feedback loop of IBD pathogenesis and thereby further exacerbates IBD activity. Thus, the ECS offers a promising new framework for identifying molecular compounds and pathways to target in IBD therapeutics. Some common avenues currently being explored include:
- inhibiting degradation of the endocannabinoids by altering levels of their metabolic enzymes, or
- altering cannabinoid receptor activity with system antagonists (which decrease ECS activity) and agonists (which increase ECS activity).
Phytocannabinoids from medical cannabis have been shown to exert effects opposite to those exerted by endocannabinoids on IBD activity and have thus far provided lots of promising potential for providing symptom relief to IBD patients. However, much more research is needed to determine the full spectrum of effects of phytocannabinoids on IBD pathophysiology in humans.
Key Takeaways
Although all of the mechanisms underlying the role of the ECS in IBD are not yet fully elucidated, it is clear that crosstalk between the ECS and microbiota-gut-brain axis plays a critical role in regulating the three molecular mechanisms of IBD pathogenesis. Dysbiosis, gut permeability, and inflammation can all be influenced by both endogenous and exogenous cannabinoids.
The gut ECS has been shown to regulate GI motility, barrier permeability, inflammation, stress responses, and metabolic processes through bi-directional crosstalk along the MGBA. Thus, ECS activity influences and is influenced by metabolic state (i.e., fasting vs. feeding), metabolic conditions (i.e., diet, probiotic/antibiotic exposure), and inflammatory state through the MGBA. Furthermore, microbial composition and ECS activity are also able to bi-directionally modulate one another.
When disrupted, the ECS can be a source of increased IBD activity and emotional dysregulation. When in balance, the ECS can be a positive force for change to help promote remission and management of symptoms of IBD. To keep the ECS in check, it is important to nurture a healthy relationship between the ECS and MGBA. This can be achieved through lifestyle interventions such as diet (i.e., avoiding high-fat, carbohydrate-rich foods; eating small meals of gut-healthy foods throughout the day), stress management techniques (i.e., meditation, practicing mindfulness), prebiotics, probiotics, and positive-thinking practices.
Here at IBDCoach, we strongly believe in empowering people with IBD to understand how different biological systems within their bodies – like the ECS and MGBA – contribute to their health, disease, and responsiveness to particular treatments. It is important to remember that there is no cure-all for IBD and that each person’s journey might look a little different because achieving remission requires taking a holistic approach to treatment that involves a combination of dietary and lifestyle changes in conjunction with medication.
Ready to harness your own knowledge to generate a personalized plan for achieving long-lasting remission from your IBD? Enroll in our FREE online Microcourse: The Foundations of Remission to learn more.
- Srivastava RK, Lutz B, Ruiz de Azua I. The Microbiome and Gut Endocannabinoid System in the Regulation of Stress Responses and Metabolism. Front Cell Neurosci. 2022;16. https://www.frontiersin.org/article/10.3389/fncel.2022.867267
- Sharkey KA, Wiley JW. The Role of the Endocannabinoid System in the Brain-Gut Axis. Gastroenterology. 2016;151(2):252-266. doi:10.1053/j.gastro.2016.04.015
- Liu RH. Potential Synergy of Phytochemicals in Cancer Prevention: Mechanism of Action. J Nutr. 2004;134(12):3479S-3485S.
- Cocetta V, Governa P, Borgonetti V, et al. Cannabidiol Isolated From Cannabis sativa L. Protects Intestinal Barrier From In Vitro Inflammation and Oxidative Stress. Front Pharmacol. 2021;12. https://www.frontiersin.org/article/10.3389/fphar.2021.641210
- Borrelli F, Aviello G, Romano B, et al. Cannabidiol, a safe and non-psychotropic ingredient of the marijuana plant Cannabis sativa, is protective in a murine model of colitis. J Mol Med. 2009;87(11):1111.
- Vučković S, Srebro D, Vujović KS, Vučetić Č, Prostran M. Cannabinoids and Pain: New Insights From Old Molecules. Front Pharmacol. 2018;9. https://www.frontiersin.org/article/10.3389/fphar.2018.01259
- Russo EB. Clinical Endocannabinoid Deficiency Reconsidered: Current Research Supports the Theory in Migraine, Fibromyalgia, Irritable Bowel, and Other Treatment-Resistant Syndromes. Cannabis Cannabinoid Res. 2016;1(1):154-165.
- Alhamoruni A, Wright KL, Larvin M, O’Sullivan SE. Cannabinoids mediate opposing effects on inflammation-induced intestinal permeability. Br J Pharmacol. 2012;165(8):2598-2610.
- Alhamoruni A, Lee AC, Wright KL, Larvin M, O’Sullivan SE. Pharmacological effects of cannabinoids on the Caco-2 cell culture model of intestinal permeability. J Pharmacol Exp Ther. 2010;335(1):92-102.
- Michielan A, D’Incà R. Intestinal Permeability in Inflammatory Bowel Disease: Pathogenesis, Clinical Evaluation, and Therapy of Leaky Gut. Mediators Inflamm. 2015;2015:628157. doi:10.1155/2015/628157
- Chen T, Kim CY, Kaur A, et al. Dietary fibre-based SCFA mixtures promote both protection and repair of intestinal epithelial barrier function in a Caco-2 cell model. Food Funct. 2017;8(3):1166-1173.
- Thursby E, Juge N. Introduction to the human gut microbiota. Biochem J. 2017;474(11):1823-1836. doi:10.1042/BCJ20160510
- Martin-Gallausiaux C, Marinelli L, Blottière HM, Larraufie P, Lapaque N. SCFA: mechanisms and functional importance in the gut. Proc Nutr Soc. 2021;80(1):37-49. doi:10.1017/S0029665120006916
- D’argenio G, Valenti M, Scaglione G, Cosenza V, Sorrentini I, Di Marzo V. Up‐regulation of anandamide levels as an endogenous mechanism and a pharmacological strategy to limit colon inflammation. FASEB J. 2006;20(3):568-570.
- Bruewer M, Samarin S, Nusrat A. Inflammatory Bowel Disease and the Apical Junctional Complex. Ann N Y Acad Sci. 2006;1072(1):242-252.
- Amasheh S, Milatz S, Krug SM, et al. Tight Junction Proteins as Channel Formers and Barrier Builders. Ann N Y Acad Sci. 2009;1165(1):211-219.
- Massa F, Marsicano G, Hermann H, et al. The endogenous cannabinoid system protects against colonic inflammation. J Clin Invest. 2004;113(8):1202-1209.
- Gassler N, Rohr C, Schneider A, et al. Inflammatory bowel disease is associated with changes of enterocytic junctions. Am J Physiol-Gastrointest Liver Physiol. 2001;281(1):G216-G228.
- Muccioli GG, Naslain D, Bäckhed F, et al. The endocannabinoid system links gut microbiota to adipogenesis. Mol Syst Biol. 2010;6(1):392.
- Appleton J. The Gut-Brain Axis: Influence of Microbiota on Mood and Mental Health. Integr Med Encinitas Calif. 2018;17(4):28-32.
- Picciotto MR. Galanin–25 years with a multitalented neuropeptide. Cell Mol Life Sci. 2008;65(12):1872-1879.
- Renthal W. Chapter 23 – Pain genetics. Rosenberg RN, Pascual JM, eds. Rosenb Mol Genet Basis Neurol Psychiatr Dis Sixth Ed. Published online 2020:397-410. https://www.sciencedirect.com/science/article/pii/B9780128138663000230
- Yang G, Wei J, Liu P, et al. Role of the gut microbiota in type 2 diabetes and related diseases. Metabolism. 2021;117:154712.
- Scheithauer TPM, Rampanelli E, Nieuwdorp M, et al. Gut Microbiota as a Trigger for Metabolic Inflammation in Obesity and Type 2 Diabetes. Front Immunol. 2020;11. https://www.frontiersin.org/article/10.3389/fimmu.2020.571731
- Cani PD. Microbiota and metabolites in metabolic diseases. Nat Rev Endocrinol. 2019;15(2):69-70.
- Everard A, Belzer C, Geurts L, et al. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc Natl Acad Sci. 2013;110(22):9066-9071.
- Grider JR, Mahavadi S, Li Y, et al. Modulation of motor and sensory pathways of the peristaltic reflex by cannabinoids. Am J Physiol-Gastrointest Liver Physiol. 2009;297(3):G539-G549.
- Guida F, Turco F, Iannotta M, et al. Antibiotic-induced microbiota perturbation causes gut endocannabinoidome changes, hippocampal neuroglial reorganization and depression in mice. Brain Behav Immun. 2018;67:230-245.
- Storr M, Emmerdinger D, Diegelmann J, et al. The Cannabinoid 1 Receptor (CNR1) 1359 G/A Polymorphism Modulates Susceptibility to Ulcerative Colitis and the Phenotype in Crohn’s Disease. PLOS ONE. 2010;5(2):e9453.
- Storr M, Emmerdinger D, Diegelmann J, et al. The role of fatty acid hydrolase gene variants in inflammatory bowel disease. Aliment Pharmacol Ther. 2009;29(5):542-551.
- Suárez J, Romero-Zerbo Y, Márquez L, et al. Ulcerative colitis impairs the acylethanolamide-based anti-inflammatory system reversal by 5-aminosalicylic acid and glucocorticoids. Plos One. 2012;7(5):e37729.
- Di Sabatino A, Battista N, Biancheri P, et al. The endogenous cannabinoid system in the gut of patients with inflammatory bowel disease. Mucosal Immunol. 2011;4(5):574-583.
- Marquéz L, Suárez J, Iglesias M, Bermudez-Silva FJ, Rodríguez de Fonseca F, Andreu M. Ulcerative colitis induces changes on the expression of the endocannabinoid system in the human colonic tissue. PloS One. 2009;4(9):e6893.
- Sałaga M, Mokrowiecka A, Zakrzewski PK, et al. Experimental colitis in mice is attenuated by changes in the levels of endocannabinoid metabolites induced by selective inhibition of fatty acid amide hydrolase (FAAH). J Crohns Colitis. 2014;8(9):998-1009.
- Berland C, Castel J, Terrasi R, et al. Identification of an endocannabinoid gut-brain vagal mechanism controlling food reward and energy homeostasis. Mol Psychiatry. 2022;27(4):2340-2354.
- Egerod KL, Petersen N, Timshel PN, et al. Profiling of G protein-coupled receptors in vagal afferents reveals novel gut-to-brain sensing mechanisms. Mol Metab. 2018;12:62-75.
- Vianna CR, Donato J, Rossi J, et al. Cannabinoid Receptor 1 in the Vagus Nerve Is Dispensable for Body Weight Homeostasis But Required for Normal Gastrointestinal Motility. J Neurosci. 2012;32(30):10331.





