What is the Microbiome-Gut-Brain Axis?
The human gut microbiome is one of the hottest topics in health-related research right now. The gut (particularly the colon) is inhabited by an average of 100 trillion microbes hailing from 1000 species.1 While “microbiome” technically refers to the sum of genetic material contained by these organisms, the term “gut microbiome” is commonly used synonymously with “gut microbiota” to refer to the collection of microbes living within the gut. The microbiome-gut-brain axis (MGBA) is a robust functional network between the gut and the brain that universally influences human health and disease through complicated crosstalk between the digestive, endocrine, immune, and autonomic nervous systems.2,3
Not only do the microbiota and their collective genetic material play a central role in gut health, but emerging evidence indicates they also greatly influence our whole-body health, brain, and state of mind. Dysbiosis, an alteration of the composition and function of the gut microbiota, has been increasingly identified in many human ailments, including in people with inflammatory bowel diseases (IBD) like Crohn’s disease, ulcerative colitis, and microscopic colitis.4
The Microbiome in Human Health and Disease
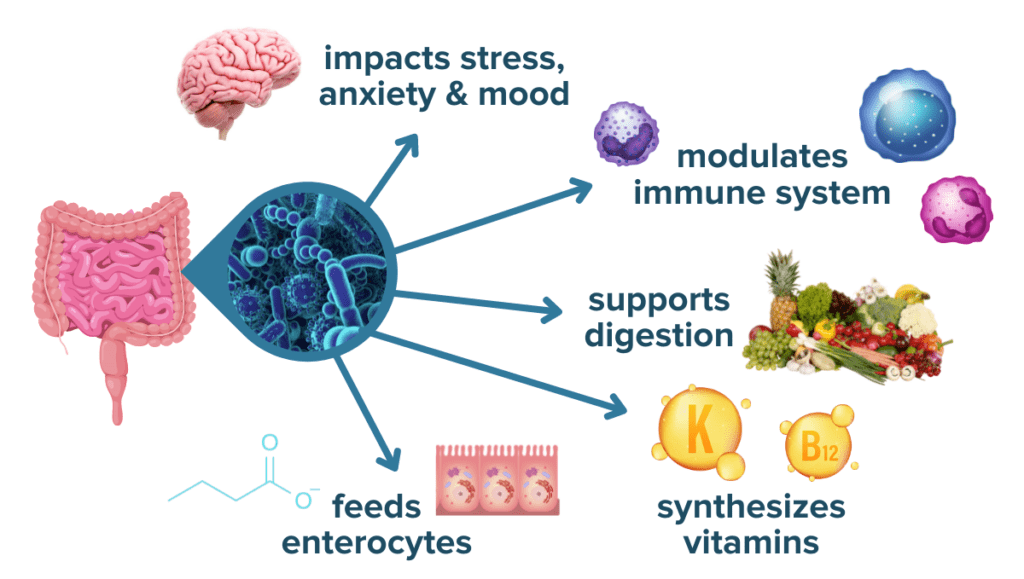
Our microbiota, which can be described as our microbial “organ,” serves various roles including:
- regulating our immunity
- protecting us against pathogenic infection,
- harvesting energy from our food to create beneficial metabolites like short-chain fatty acids (SCFAs) and some vitamins
- modulating activation of our stress response
- maintaining our intestinal barrier’s integrity.5
Learn How Flavonoids Help Fuel a Balanced Microbiome
We are exposed to microorganisms from birth and continue to be colonized by more and more diverse microbes throughout the first few years of life. By age 3-5, our microbiota more closely resembles the population that will persist through our adult lives.5 The microbiota may be influenced over time, however, by environmental factors including stress, diet, and antibiotic use. Because of the many contextual factors that influence the human gut microbiome, the gut microbiome is incredibly diverse and varies dramatically from person to person: even identical twins only demonstrate an average of 50% overlap in microbial similarity.6 Collectively, these microbes in the microbiome-gut-brain axis have widespread impacts on human health and disease.
The Microbiome in Whole-Body Health
All elements of human health are connected: the human body is a complex system comprised of millions of moving parts interacting bidirectionally with the environment around it. The essential functions of the gut microbiome underscore how delicate the human system is and how easily it is for the system to become misaligned. The gut microbiome plays an essential role in vitamin and nutrient metabolism (vitamin K and B) and in the development and maintenance of the immune and central nervous systems.7 Dysbiosis in the intestinal microbiome has been correlated to countless human physiological conditions through those functions: nutrition status, food allergies, obesity, diabetes, liver function, cardiovascular function, immune-related disorders, autoimmunity, depression, anxiety, Alzheimer’s disease, Parkinson’s disease, and many more. Some of the strongest ties between the microbiome-gut-brain axis and physiological systems have been observed in conditions known to have a stress-related, psychological, or cognitive component such as obesity, irritable bowel syndrome, or inflammatory bowel disease.8
The Microbiome and Brain Health and Behavior
Recent studies have investigated how the trillions of bacteria in the gut can modulate human brain function and behavior. The impact of gut microbes on our development can be observed in “germ-free” mice (mice stripped of their microbiomes): their brains have significant deviations from normal mice in terms of brain volume and myelination (insulation of neurons). Functional deficits are also observed in anxiety-like behaviors, memory, sociability, and depressiveness.9,10 Both animal and human studies have demonstrated that individuals with depression present a microbial imbalance hallmarked by a reduction in microbial richness and diversity.11,12 Further, one mouse study found that dietary changes increased microbial diversity and were associated with improved cognitive ability.13 It is clear that our microbiota not only plays an essential role in whole-body health but also in brain physiology and subsequent behavior.
Brain-imaging studies of the MGBA as far back as the 1980s demonstrate a direct connection between gut disturbances and resulting brain stimulus, such as areas in the brain that become activated due to gut distention (increased abdominal girth often from excess intestinal gas or fluid.) These effects were more pronounced in those with GI diseases.8 We now know that signals from the gut can reach different parts of the brain including the limbic system, the prefrontal cortex, the amygdala, the hippocampus, and the cingulate cortex, all of which could influence our self-awareness, morality, fear, memory, and motivation.14 Emerging evidence on the many connections of the microbiome-gut-brain axis only further supports its link to IBD pathogenesis and treatment outcomes.
The Microbiome-Gut-Brain-Axis and Inflammatory Bowel Disease
The gut microbiota and brain are remarkably interconnected systems of the human body that are plastic, moldable entities. Because the MGBA has such substantial, widespread implications in human health and disease, understanding the functions of the microbiome-gut-brain axis as they relate to IBDs like Crohn’s disease, ulcerative colitis, and microscopic colitis may be beneficial for patients looking to take better control of their condition.
The Microbiome and IBD Risk
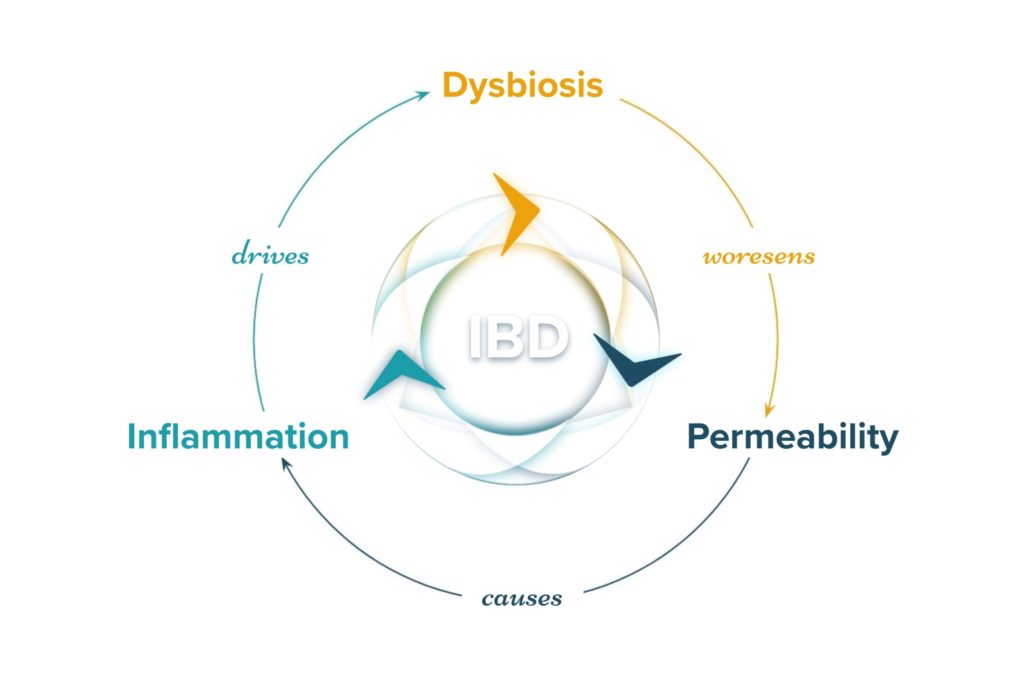
Before understanding how the power of the MGBA can be harnessed to affect inflammatory bowel disease outcomes, it is important to understand how dysbiosis in the microbiome-gut-brain axis may first influence the risk of developing IBD and its pathogenesis.15 Numerous studies comparing humans with IBD to those without have demonstrated a consistent link between microbial dysbiosis, decreased microbial diversity, and increased risk for developing IBD. Unique microbiome signatures have been identified that are associated with UC, CD, and their respective subtypes.16 Additionally, studies have identified certain dietary patterns that are known to increase the risk of developing IBD such as the high fat, high added sugar, and low fiber Standard American Diet.17 The Standard American Diet itself has been further associated with causing dysbiosis and disruptions to the microbiota-gut-brain axis and immunity.18 Dysbiosis of the microbiome is one of the three central mechanisms that drive IBD and the symptoms of IBD.
Learn More About the Science Behind IBD
The Microbiome and Physiological Symptoms of IBD
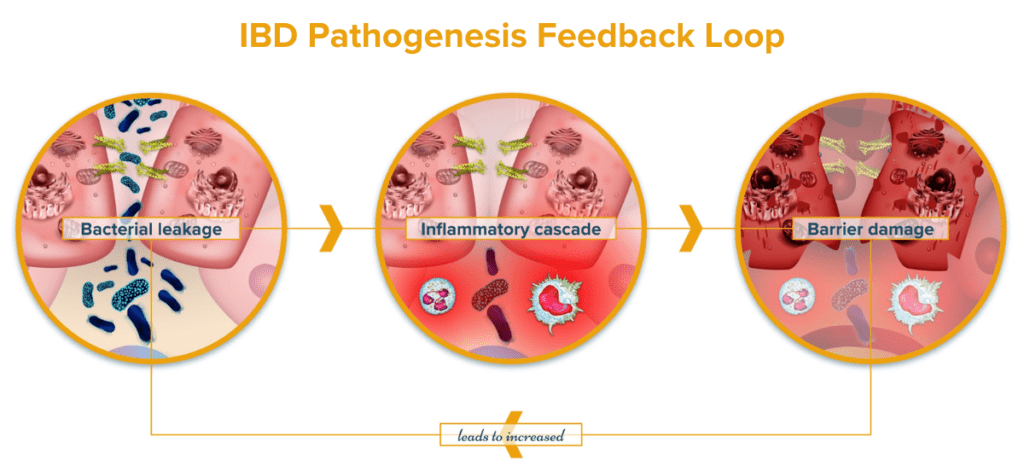
Microbes play essential roles in maintaining homeostasis in the GI tract. Reductions in microbial diversity result in increased intestinal inflammation and lesions. Microbes are known to play a major role in regulating the immune system and maintaining healthy gut immunity.19 Microbes are known to provide essential nutrients like the SCFA butyrate to colonocytes via microbial fermentation that contribute to a strong and healthy intestinal lining.20 A weak intestinal lining increases barrier permeability (“leaky gut”) which can allow microbes and other bacterial particulates to translocate across the intestinal barrier. When this happens, an immune response is initiated against the microbes and bacterial particulates that crossed the barrier. This creates a runaway cycle of increasing IBD activity: dysbiosis leads to increased intestinal permeability resulting in inflammation and immune activity which further promotes dysbiosis.21 Additional studies have also concluded that the microbial changes observed in IBD result in functional changes of the microbiome, like decreased vitamin K synthesis, that may exacerbate IBD symptoms.22 Some of the connections between the microbiome and symptoms of IBD highlights the role that the brain plays in this complicated system.
The Microbiome and the Brain in IBD
Our brain, the other incredible organ involved in the MGBA, has over 80 billion neurons that are continuously communicating with each other through electrical signals and chemical messengers called neurotransmitters. For centuries, the brain was considered fixed or “hard-wired” after adolescence, but thanks to recent advances in neuroscience, we now understand that neuroplasticity–the brain’s ability to change and adapt as a response to our internal and external environment–persists long into adulthood.23 Through the microbiome-gut-brain axis, both physical and psychological stress has been linked to increased intestinal permeability.24 Studies confirm that psychological stress increases symptom prevalence and exacerbation in IBD by disturbing the intestinal microbiota and triggering an immune system response.25,26 In fact, people with IBD tend to have higher rates of psychological abnormalities compared to people with other chronic diseases.27 Considering that intestinal permeability and an overt immune response are the hallmarks of IBD, managing stress levels to enact positive change on the activity of the microbiome-gut-brain axis may be a promising avenue by which people with IBD can positively impact their gut and brain health.
Read About The Surprising Connection Between the MGBA, Psychedelics & IBD
The microbiome is known to be malleable, influenced by diet, stress, genetics, antibiotics, exercise, disease, and the environment. Knowing dysbiosis is often found in IBD patients and may relate to disease risk and pathogenesis, is it possible to change this ecosystem and its effects on the brain to profoundly change the lives of IBD patients?
The Microbiome and IBD Treatments
How is this growing wealth of knowledge being applied to current IBD treatment approaches? Numerous strategies to alleviate symptoms of IBD, from dietary plans targeted at influencing the microbiome to fecal microbial transplants (yes, poop transplants), have gained popularity in recent years. While none of these strategies alone are sufficient to result in IBD remission, many of these strategies may be valuable tools in a rounded IBD treatment protocol that has been approved by your physicians.
Read Our Comprehensive Review of IBD Treatments
Diet and IBD
The food a human eats is food and fuel for their gut microbes, and that means diet is one of the easiest and most direct ways to influence the microbiome-gut-brain axis in inflammatory bowel disease.28 Microbiome researchers are beginning to gain a deeper understanding of which food sources are preferred by different gut microbes.29 Some gut microbes are (generally) associated with more positive health effects while others are (generally) associated with more negative health effects, so it stands to reason that prioritizing the foods preferred by the beneficial bacteria would help them flourish while starving out the pathogenic competitors. It is important to note that the health effects of microbiota vary dramatically depending on the contextual and environmental factors unique to each individual, so it is impossible to develop a single set of definitive rules about which foods will feed good or bad microbes. Another interesting recent study demonstrated that the adaptive immune system responds to bacterial antigens that are modulated by dietary metabolites.30 Overall, this growing body of scientific research suggests that dietary alterations could be a mechanism by which people with immune-mediated conditions like IBD can help influence the microbiome and subsequent immune activity and disease activity.
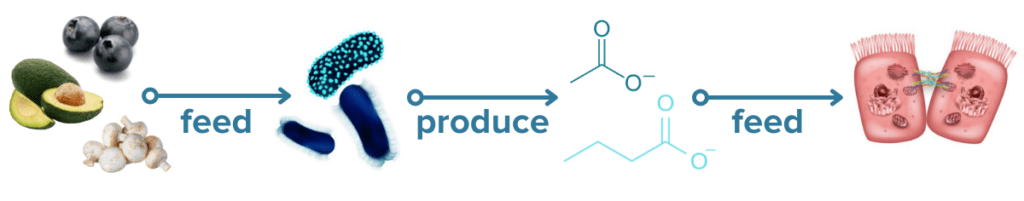
Probiotics and IBD
Probiotics are a kind of supplement containing live microbes that are generally regarded as beneficial for gut health. Because of the strong links between the microbiome-gut-brain axis and IBD, probiotics are an extremely popular area of research for IBD treatments and one of the first complementary treatment strategies that many people with IBD consider. While the microbes that are found in probiotic supplements and probiotic-containing foods yogurt or kimchi likely do not take up long term residence in the gut, they can confer many health benefits as they pass through the digestive tract such as producing beneficial metabolites and aiding digestion.31
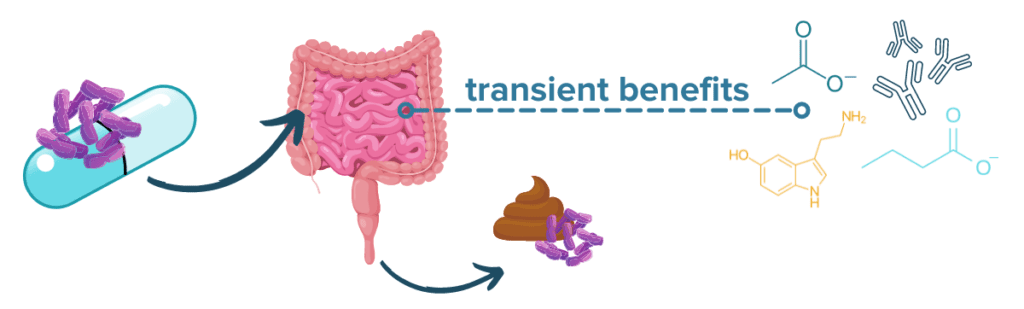
The scientific evidence in humans is currently mixed and somewhat limited, but some studies suggest that certain probiotics may have beneficial effects on IBD symptoms (especially in ulcerative colitis) and brain health. The research in other fields has been promising: early evidence even suggests that alterations to the gut microbiota using prebiotics (microbiota foods like fiber) and/or probiotics may attenuate neuropsychiatric symptoms.32,33 Additionally, specific probiotic formulations like Visbiome® (formerly known as VSL#3) may be able to induce remission in people with UC.34 The effects of probiotics are strain-dependent, so it is important both to check with a physician and to understand the benefits of the specific strains present in a particular probiotic product before trying it out. As a helpful analogy, you can think of strains like breeds of dogs: all dogs are technically the same species, but if you accidentally wind up with a Great Dane when you were trying to adopt a Chihuahua, you’ll see that breed (or strain) matters quite a bit.
FMT and IBD
Fecal microbial transplant, or FMT, is on the cutting edge of treatments for IBDs like Crohn’s disease and ulcerative colitis. FMT works by introducing new populations of microbes from a healthy donor to the gut of an IBD patient. While FMT cannot cure a chronic condition like CD or UC, a recent meta-analysis revealed that as many as 33% of IBD patients may achieve remission following FMT.35 Studies have varied widely, however, with rates of remission ranging from 10% to over 80%.36 Researchers are still working to refine the technology and understand the effects of disease location, co-treatments, and mode of delivery on the success rate of FMT.
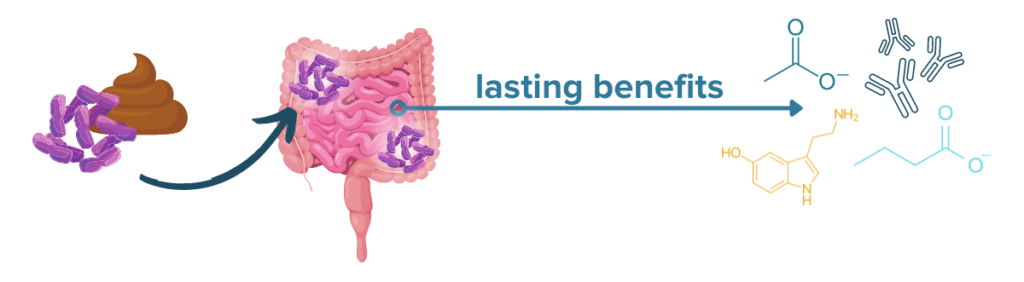
FMT treatment may be especially effective in IBD patients with colonic disease activity and recurrent C. difficile infection (CDI). One 2020 study and a recent analysis found that recurrent CDI was cured by FMT in 80% of the IBDers tested and that 73.3% of CD patients and 62% of UC patients with recurrent CDI also experienced an improvement in IBD symptoms following FMT.37,38 Several clinical trials of FMT for IBD are ongoing, but it is not yet formally approved for widespread use in the US, and home-based or DIY FMT methods are unsafe and dangerous.
Rewilding the Microbiome and IBD
An emerging theory on rebalancing the microbiome to recover from a state of dysbiosis is known as “rewilding” the microbiome.39 This theory is somewhat rooted in the hygiene hypothesis which suggests that many immune-related diseases may arise in part from an excess of cleanliness in modern societies: over-sanitization, abundant antibiotic use, and an overall decrease in exposure to environmental microbes. Exposure to microbes and germs is a healthy and normal part of maintaining our immune system and gut microbiota, and the act of being “too clean” may end up sabotaging our immune system. Spending time with your pet, adventuring outside, or even meditating in the park can all aid in the process of rewilding your microbiome and support a stronger immune system.40
The Microbiome and Inflammatory Bowel Disease: Takeaways
While we continue to develop our understanding of the exact role of the microbiome-gut-brain axis in IBD, it is clear that there is a strong relationship between our gastrointestinal tract, the superorganisms within it, our brain and behavior, and our health.
The MGBA is a highly interconnected and delicate system that can either drive health and wellbeing or drive increased disease activity and distress in IBD. The collection of research on the relationship between the microbiome-gut-brain axis and inflammatory bowel disease suggests that the power of the microbiome can be harnessed to help create an ecosystem and environment conducive to healing. Through methods like altering our mindset toward positivity, managing stress, prioritizing mental and emotional wellbeing, developing a diet supportive of beneficial gut microbes and gut health, and more, the microbiome-gut-brain ecosystem can be a positive force for change. It is crucial to remember, however, that the MGBA can just as easily fall out of balance and trigger a negative chain reaction: rising stress levels may result in increased gut permeability and increased immune reactivity that may consequently further diminish mood. Rising stress may then alter food choices or nutrient absorption which (in combination with the rising stress hormones) may disturb the balance of gut microbes. Altogether, this may trigger inflammatory pathways and exacerbate IBD symptoms to result in increased stress, restarting the cycle anew. Understanding how to disrupt these cycles and steer the microbiome back toward a positive, balanced system can be a powerful part of an individualized IBD treatment protocol.
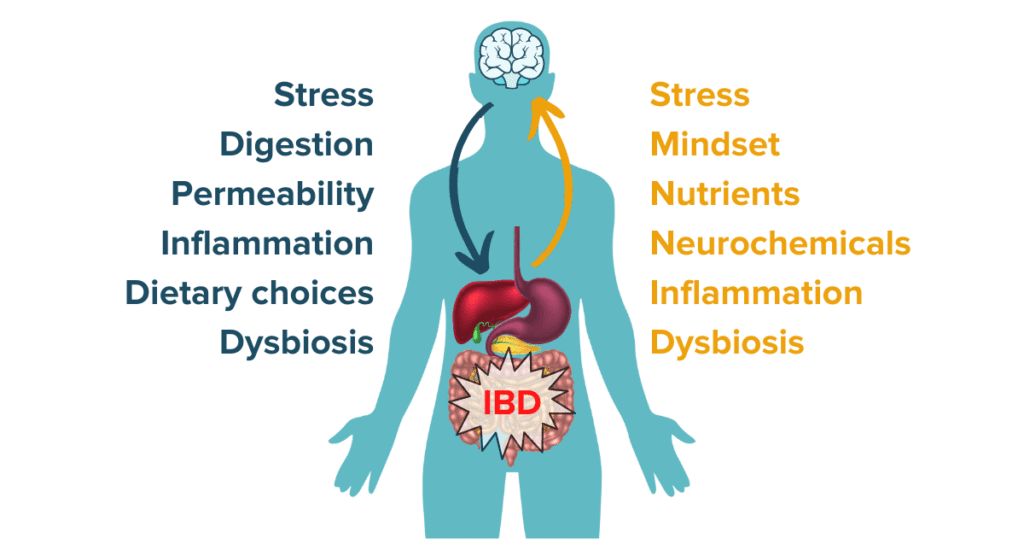
Here at IBDCoach, we believe in empowering people with IBD to understand how factors like the MGBA can influence their health and disease. We believe lifestyle interventions such as diet, meditation, prebiotics, exercise, sleep, etc. can biologically, through the microbiome-gut-brain axis, provide significant transformations in your quality of life.
Ready to harness the power of your microbiome as a part of a rounded, individualized protocol to achieve robust remission from your IBD? Enroll in our FREE online Microcourse: The Foundations of Remission to learn more.
- Guinane, C. M. & Cotter, P. D. Role of the gut microbiota in health and chronic gastrointestinal disease: understanding a hidden metabolic organ. Ther. Adv. Gastroenterol. 6, 295–308 (2013).
- Bray, N. The microbiota–gut–brain axis. Nat. Res. (2019) doi:10.1038/d42859-019-00021-3.
- Sinagra, E. et al. Microbiota-gut-brain axis and its affect inflammatory bowel disease: Pathophysiological concepts and insights for clinicians. World J. Clin. Cases 8, 1013–1025 (2020).
- Nishida, A. et al. Gut microbiota in the pathogenesis of inflammatory bowel disease. Clin. J. Gastroenterol. 11, 1–10 (2018).
- Thursby, E. & Juge, N. Introduction to the human gut microbiota. Biochem. J. 474, 1823–1836 (2017).
- Turnbaugh, P. J. et al. Organismal, genetic, and transcriptional variation in the deeply sequenced gut microbiomes of identical twins. Proc. Natl. Acad. Sci. U. S. A. 107, 7503–7508 (2010).
- Schächtle, M. A. & Rosshart, S. P. The Microbiota-Gut-Brain Axis in Health and Disease and Its Implications for Translational Research. Front. Cell. Neurosci. 15, (2021).
- Cryan, J. F. et al. The Microbiota-Gut-Brain Axis. Physiol. Rev. 99, 1877–2013 (2019).
- Liu, W.-H. et al. Alteration of behavior and monoamine levels attributable to Lactobacillus plantarum PS128 in germ-free mice. Behav. Brain Res. 298, 202–209 (2016).
- Campos, A. C. et al. Absence of gut microbiota influences lipopolysaccharide-induced behavioral changes in mice. Behav. Brain Res. 312, 186–194 (2016).
- Kelly, J. R. et al. Transferring the blues: Depression-associated gut microbiota induces neurobehavioural changes in the rat. J. Psychiatr. Res. 82, 109–118 (2016).
- Zheng, P. et al. Gut microbiome remodeling induces depressive-like behaviors through a pathway mediated by the host’s metabolism. Mol. Psychiatry 21, 786–796 (2016).
- Li, W., Dowd, S. E., Scurlock, B., Acosta-Martinez, V. & Lyte, M. Memory and learning behavior in mice is temporally associated with diet-induced alterations in gut bacteria. Physiol. Behav. 96, 557–567 (2009).
- Enders, G., Enders, J. & Shaw, D. Gut: the inside story of our body’s most underrated organ. (Greystone Books, 2015).
- Zuo, T. & Ng, S. C. The Gut Microbiota in the Pathogenesis and Therapeutics of Inflammatory Bowel Disease. Front. Microbiol. 9, (2018).
- Halfvarson, J. et al. Dynamics of the human gut microbiome in inflammatory bowel disease. Nat. Microbiol. 2, 1–7 (2017).
- Owczarek, D., Rodacki, T., Domagała-Rodacka, R., Cibor, D. & Mach, T. Diet and nutritional factors in inflammatory bowel diseases. World J. Gastroenterol. 22, 895–905 (2016).
- Brown, K., DeCoffe, D., Molcan, E. & Gibson, D. L. Diet-Induced Dysbiosis of the Intestinal Microbiota and the Effects on Immunity and Disease. Nutrients 4, 1095–1119 (2012).
- Belkaid, Y. & Hand, T. Role of the Microbiota in Immunity and inflammation. Cell 157, 121–141 (2014).
- Martin-Gallausiaux, C., Marinelli, L., Blottière, H. M., Larraufie, P. & Lapaque, N. SCFA: mechanisms and functional importance in the gut. Proc. Nutr. Soc. 80, 37–49 (2021).
- Michielan, A. & D’Incà, R. Intestinal Permeability in Inflammatory Bowel Disease: Pathogenesis, Clinical Evaluation, and Therapy of Leaky Gut. Mediators Inflamm. 2015, 628157 (2015).
- Hassouneh, S. A.-D., Loftus, M. & Yooseph, S. Linking Inflammatory Bowel Disease Symptoms to Changes in the Gut Microbiome Structure and Function. Front. Microbiol. 12, (2021).
- La Rosa, C., Parolisi, R. & Bonfanti, L. Brain Structural Plasticity: From Adult Neurogenesis to Immature Neurons. Front. Neurosci. 14, (2020).
- Kelly, J. R. et al. Breaking down the barriers: the gut microbiome, intestinal permeability and stress-related psychiatric disorders. Front. Cell. Neurosci. 9, 392 (2015).
- Mawdsley, J. E. & Rampton, D. S. Psychological stress in IBD: new insights into pathogenic and therapeutic implications. Gut 54, 1481–1491 (2005).
- Gao, X. et al. Chronic stress promotes colitis by disturbing the gut microbiota and triggering immune system response. Proc. Natl. Acad. Sci. U. S. A. 115, E2960–E2969 (2018).
- Mental Health—An invisible driver of IBD cost of care. Crohn’s & Colitis Foundation https://www.crohnscolitisfoundation.org/blog/mental-health-invisible-driver-ibd-cost-care.
- Berding, K. et al. Diet and the Microbiota-Gut-Brain Axis: Sowing the Seeds of Good Mental Health. Adv. Nutr. Bethesda Md 12, 1239–1285 (2021).
- Armstrong, H., Mander, I., Zhang, Z., Armstrong, D. & Wine, E. Not All Fibers Are Born Equal; Variable Response to Dietary Fiber Subtypes in IBD. Front. Pediatr. 8, (2021).
- Wegorzewska, M. M. et al. Diet modulates colonic T cell responses by regulating the expression of a Bacteroides thetaiotaomicron antigen. Sci. Immunol. (2019) doi:10.1126/sciimmunol.aau9079.
- Roselli, M. et al. Colonization Ability and Impact on Human Gut Microbiota of Foodborne Microbes From Traditional or Probiotic-Added Fermented Foods: A Systematic Review. Front. Nutr. 8, (2021).
- Rao, A. V. et al. A randomized, double-blind, placebo-controlled pilot study of a probiotic in emotional symptoms of chronic fatigue syndrome. Gut Pathog. 1, 6 (2009).
- Pinto-Sanchez, M. I. et al. Probiotic Bifidobacterium longum NCC3001 Reduces Depression Scores and Alters Brain Activity: A Pilot Study in Patients With Irritable Bowel Syndrome. Gastroenterology 153, 448-459.e8 (2017).
- Bibiloni, R. et al. VSL#3 probiotic-mixture induces remission in patients with active ulcerative colitis. Am. J. Gastroenterol. 100, 1539–1546 (2005).
- Paramsothy, S. et al. Faecal Microbiota Transplantation for Inflammatory Bowel Disease: A Systematic Review and Meta-analysis. J. Crohns Colitis 11, 1180–1199 (2017).
- Tan, P., Li, X., Shen, J. & Feng, Q. Fecal Microbiota Transplantation for the Treatment of Inflammatory Bowel Disease: An Update. Front. Pharmacol. 11, 1409 (2020).
- Tariq, R. et al. Efficacy of Fecal Microbiota Transplantation for Recurrent C. Difficile Infection in Inflammatory Bowel Disease. Inflamm. Bowel Dis. 26, 1415–1420 (2020).
- Allegretti, J. R. et al. Inflammatory Bowel Disease Outcomes Following Fecal Microbiota Transplantation for Recurrent C. difficile Infection. Inflamm. Bowel Dis. 27, 1371–1378 (2021).
- Mills, J. G. et al. Urban habitat restoration provides a human health benefit through microbiome rewilding: the Microbiome Rewilding Hypothesis. Restor. Ecol. 25, 866–872 (2017).
- Salas Garcia, M. C., Schorr, A. R., Arnold, W., Fei, N. & Gilbert, J. A. Pets as a Novel Microbiome-Based Therapy. in Pets as Sentinels, Forecasters and Promoters of Human Health (eds. Pastorinho, M. R. & Sousa, A. C. A.) 245–267 (Springer International Publishing, 2020). doi:10.1007/978-3-030-30734-9_11.