Inflammatory bowel diseases like Crohn’s disease, ulcerative colitis, microscopic colitis, and indeterminate colitis are complicated immune-mediated conditions fueled by the interactions between genetics, the environment, the microbiome, and the brain.1 Scientific research has repeatedly demonstrated a link between symptoms of IBD, stress, the microbiome, and cognitive function.2 Could enhanced neuroplasticity, improved immune function, and interactions with the microbiome be possible effects of psychedelics like psilocybin-containing “magic” mushrooms for IBD?3
What are psychedelics?
Psychedelic compounds like lysergic acid diethylamide (LSD) and psilocybin (magic mushrooms) are a class of substances known to have hallucinogenic effects. LSD is a human-made psychedelic drug first synthesized in 1938 by Albert Hoffman that rose in popularity both recreationally and in scientific research throughout the mid 20th century. Psilocybin mushrooms, on the other hand, are among a cluster of naturally occurring sources of psychedelic compounds that have been used by humans for millennia for medicinal and spiritual purposes. The effects of psychedelics vary by substance but can include altered perception and cognition, visual alterations, feelings of euphoria, changes in emotional state, and an altered sense of self. Psychedelics have long been interesting to both individuals and scientists alike for their potential therapeutic benefits. Interestingly, future therapeutic indications for psychedelics like magic mushrooms may include Crohn’s disease, ulcerative colitis, and IBD due to the complex interactions of psychedelics with the microbiome and brain.4
History of Research on Psychedelics
Research on psychedelics blossomed throughout the 1950s and 1960s as scientists sought to understand the unique properties of this interesting class of substances. This research largely came to a halt following strict governmental regulations enacted in the 1970s and 1980s.4
The past several years, however, have witnessed a renaissance of psychedelic research as a growing body of evidence highlights the potential therapeutic benefits of psychedelics for a wide range of human ailments. Research initiatives have sprung up around the world in both publicly and privately funded research institutions like the Heffter Research Institute,5 MAPs,6 Usona Institute7 and Compass Pathways.8 In 2019, one of the greatest advances in psychedelic research came when the US Food and Drug Administration (FDA) declared two well-known psychedelics as Breakthrough Therapies: MDMA-assisted psychotherapy for PTSD and psilocybin for treatment-resistant depression.9,10 The designation of Breakthrough Therapy indicates that these psychedelics are currently some of the most promising interventions under investigation for these two conditions, respectively. Recent clinical trials have also demonstrated that psychedelics like magic mushrooms can provide persistent benefits for conditions like depression and may even promote lasting neurogenesis, supporting their potential role in improving neuroplasticity. These diverse therapeutic properties are likely due to the unique chemical properties of psychedelics.
Neuropharmacology of Psychedelics
Neuropharmacology is the study of how pharmacological substances influence the brain. Different classes of psychedelics influence distinct neurotransmitter systems and receptor subtypes to result in a vast array of potential pharmacological and therapeutic effects. In a highly simplified sense, neurotransmitters are the language that neurons (brain cells) use to communicate and stimulate brain activity.11 Much like our spoken language, the meaning and effect of any one neurotransmitter is dependent on the context of the speaker and audience. The words, “I love you,” mean very different things when spoken to your mother, your significant other, or your dog.
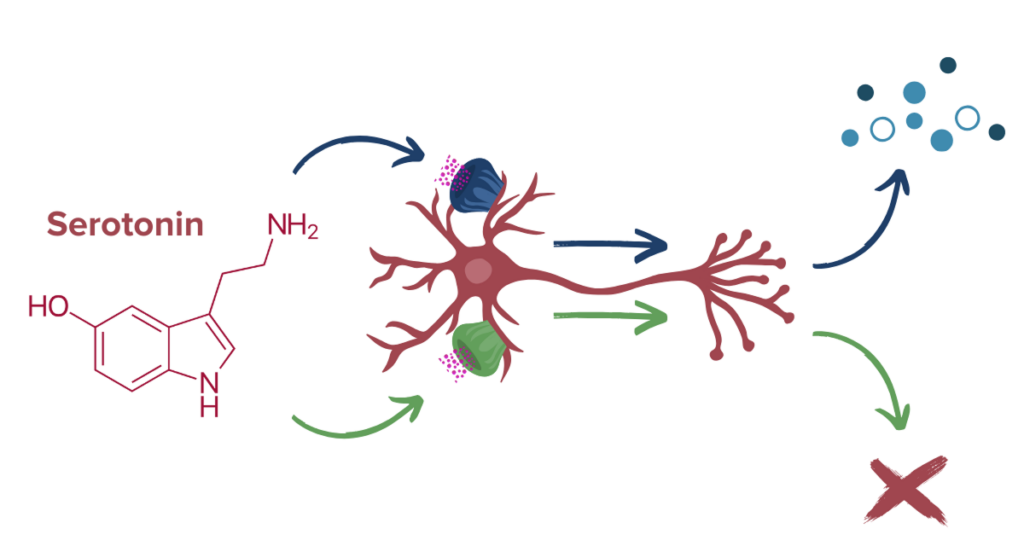
One of the primary neurotransmitter systems that psychedelics interact with is the serotonin (5-HT) system.12 Serotonin is a widespread neurotransmitter known to play significant roles in mood and emotional regulation. The brain contains numerous types of serotonin receptors that serve different functions depending on location: some serotonin receptors increase the activity of a brain cell, while others may decrease or suppress it. Additionally, serotonin is the principal neurotransmitter of the GI system. In the gut, 5-HT helps regulate gut motility, maintain intestinal permeability, and communicate with gut microbes.13,14
Ultimately, the effects of psychedelics on the brain are thought to influence something known as neuroplasticity. In the past, it was widely believed that the adult human brain is relatively static and incapable of change largely because most adult neurons cannot divide. It is now understood, however, that functional and structural changes, such as changes in the connectivity between neurons, occur in the brain throughout adulthood. This increased neuroplasticity seems to be the primary mechanism by which psychedelics confer therapeutic effects.15 Additionally, there may be some added impact on serotonin systems in the gut and on gut microbes. Let’s take a closer look at what distinguishes different classes and kinds of psychedelic compounds.
Tryptamines (LSD, magic mushrooms & DMT)
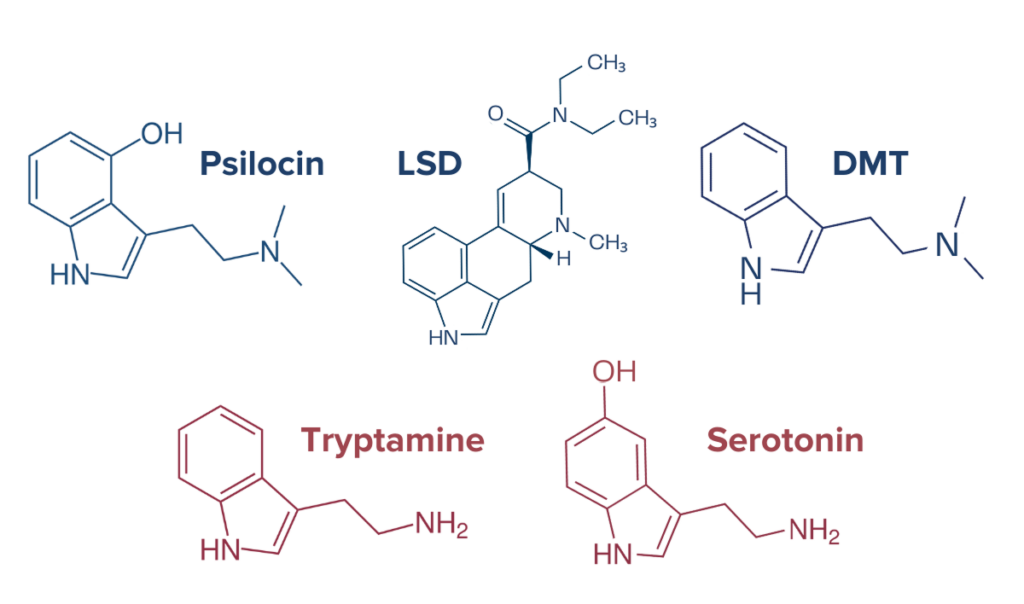
Tryptamine psychedelics, which include psilocybin and LSD, primarily interact with serotonin systems in the brain.16 Psilocybin and its active metabolite psilocin have an affinity for a broad range of serotonin receptors including 5-HT1A/B/C/D/E, 2B, 5, 6, and 7. Psilocybin and psilocin have the highest affinity for the 5-HT2A receptor. LSD has an affinity for 5-HT1A/D, 2A/B/C, and 5-HT6 receptors. Additionally, LSD can bind to the dopamine D1 and D2 receptors and α-adrenergic receptors. Dopamine is a neurotransmitter involved in processes like emotional regulation, cognition, and reward systems. DMT (N, N-dimethyltryptamine), another tryptamine, is known to activate 5-HT1A/D, 2A, and 5-HT6 receptors. Additionally, when paired with β-alkaloids, as in the case in the DMT-containing ayahuasca, DMT is capable of inhibiting monoamine oxidase A (MAO). MAO is an enzyme responsible for breaking down amine neurotransmitters like serotonin, norepinephrine, and dopamine, so inhibition of MAO by the compounds in ayahuasca can further augment the effects of these neurotransmitters. The immediate effects of tryptamine psychedelics can sustain for anywhere from 15 minutes to 12 hours, depending on blood plasma concentrations and substance consumed. But even after the psychedelic effects have subsided, studies have revealed that some tryptamine psychedelics like magic mushrooms may have lasting effects on mood and brain connectivity.
Ketamine and other Psychedelic Compounds
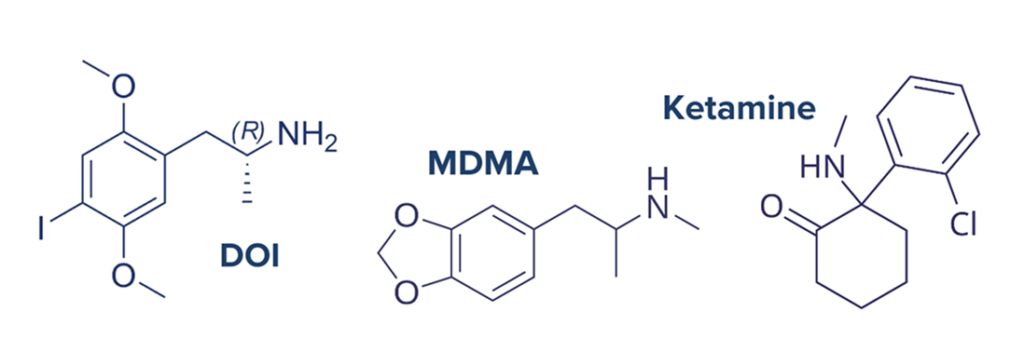
While tryptamines are some of the most common psychedelic compounds, other classes of chemicals are known to have psychedelic effects and act on slightly different pathways. Ketamine, an anesthetic compound commonly used in animals, can be psychedelic when taken by humans in certain doses. Ketamine functions as an NMDA receptor antagonist which ultimately results in elevated brain-derived neurotrophic factor (BDNF) synthesis. BDNF is a neural protein associated with neuroplasticity and neurodevelopment. Ketamine may have additional BDNF bolstering effects through AMPA receptor agonism in the neural cortex. Like other psychedelics, this indicates the potential of ketamine to induce lasting changes in brain function and connectivity.12 Other classes of psychedelic compounds include phenethylamines like (3,4-methylenedioxy-N-methylamphetamine (MDMA) and the naturally occurring compound mescaline. and DOI17 MDMA primarily acts in the brain by blocking serotonin-reuptake by neurons which effectively increases the amount of serotonin available for signaling in the brain.18
The Impact of Set & Setting on Psychedelics
In addition to the direct biological effects of psychedelic compounds on neural activity, factors like mental state (set), the environment in which a substance is consumed (setting), mood, and personality can have major effects on the outcome of an experience with psychedelics.12 Ever heard of a “bad trip”? There may be something to it. Studies have confirmed that mindset plays a major role in treatment outcomes for conventional medications. Being in a positive space (both physically and mentally) may have a major impact on the outcome of using psychedelics therapeutically. In addition to mindset and setting being especially important factors of using psychedelics as a therapeutic agent, there are several other key differences between psychedelics and standard pharmaceuticals to consider.
Psychedelics vs. Traditional Pharmaceutical Treatments for IBD
Psychedelics and pharmaceutical treatments for IBD are two entirely different things. Pharmaceutical treatments for IBD are formally approved medications that have gone through rigorous clinical trials to assess their efficacy and safety specifically for use in inflammatory bowel disease. IBD medications are effective at reducing inflammation and controlling symptoms of IBD, and psychedelics are NOT a suitable replacement for any approved IBD treatments. Other differences between psychedelics and traditional pharmaceuticals include their mechanisms of action and duration of effect.
Many traditional pharmaceuticals deliver acute effects and need to be taken regularly to maintain activity (such as monthly infusions or daily pills). Psychedelics, however, have been observed to confer lasting benefits that extend far beyond the time of administration. Studies have shown, for example, that self-administration of psilocybin or ayahuasca (a natural compound that contains DMT) in a social setting resulted in positive emotional and cognitive benefits that lasted for up to 4 weeks after consumption of the substance, even though the psychedelic remains in the blood for only several hours. Psilocybin mushrooms have also been demonstrated to induce lasting increases in neuroplasticity. 19
Before we can begin to understand how these fascinatingly diverse and unique compounds could someday become promising therapeutics for use in IBD, it is important to understand the biology behind IBD and its complex interactions with the immune system, microbiome, and brain.
What is IBD?
The inflammatory bowel diseases (IBDs) are idiopathic & chronic immune-mediated conditions that affect all or specific parts of the gastrointestinal tract. A dysregulated immune response in the mucosal membrane of the GI tract results in inflammation of the tissue and subsequent symptoms and complications. IBD is considered idiopathic because there is no clear cause of the condition, though it seems most likely that IBD arises from a complex interaction between genetic risk, diet, the gut microbiome, stress, and other environmental factors. While many medications are effective at controlling symptoms of IBD, there is currently no cure for the condition. Understanding the unique factors that drive the development and progression of IBD may help better understand why psychedelics may be on the horizon for use in this condition.
Read More About Inflammatory Bowel Disease
The Immune System and Inflammation in IBD
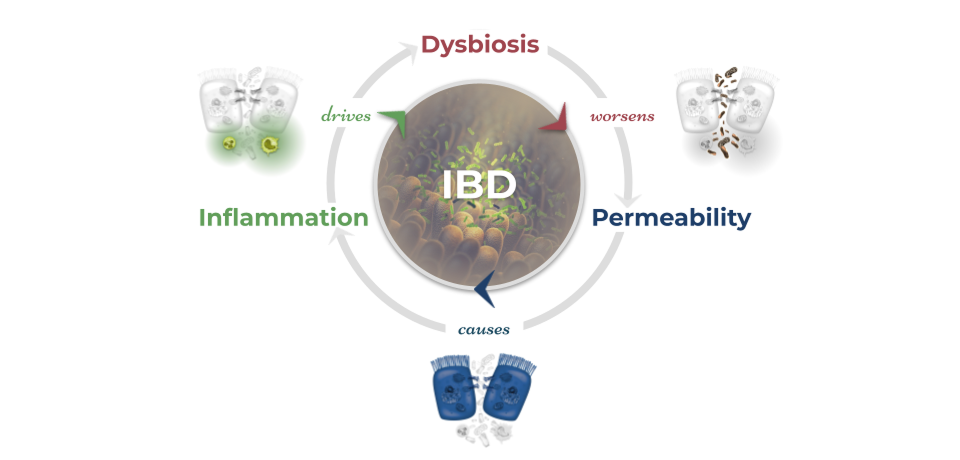
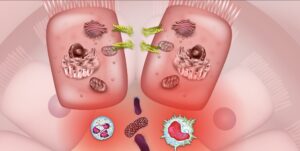
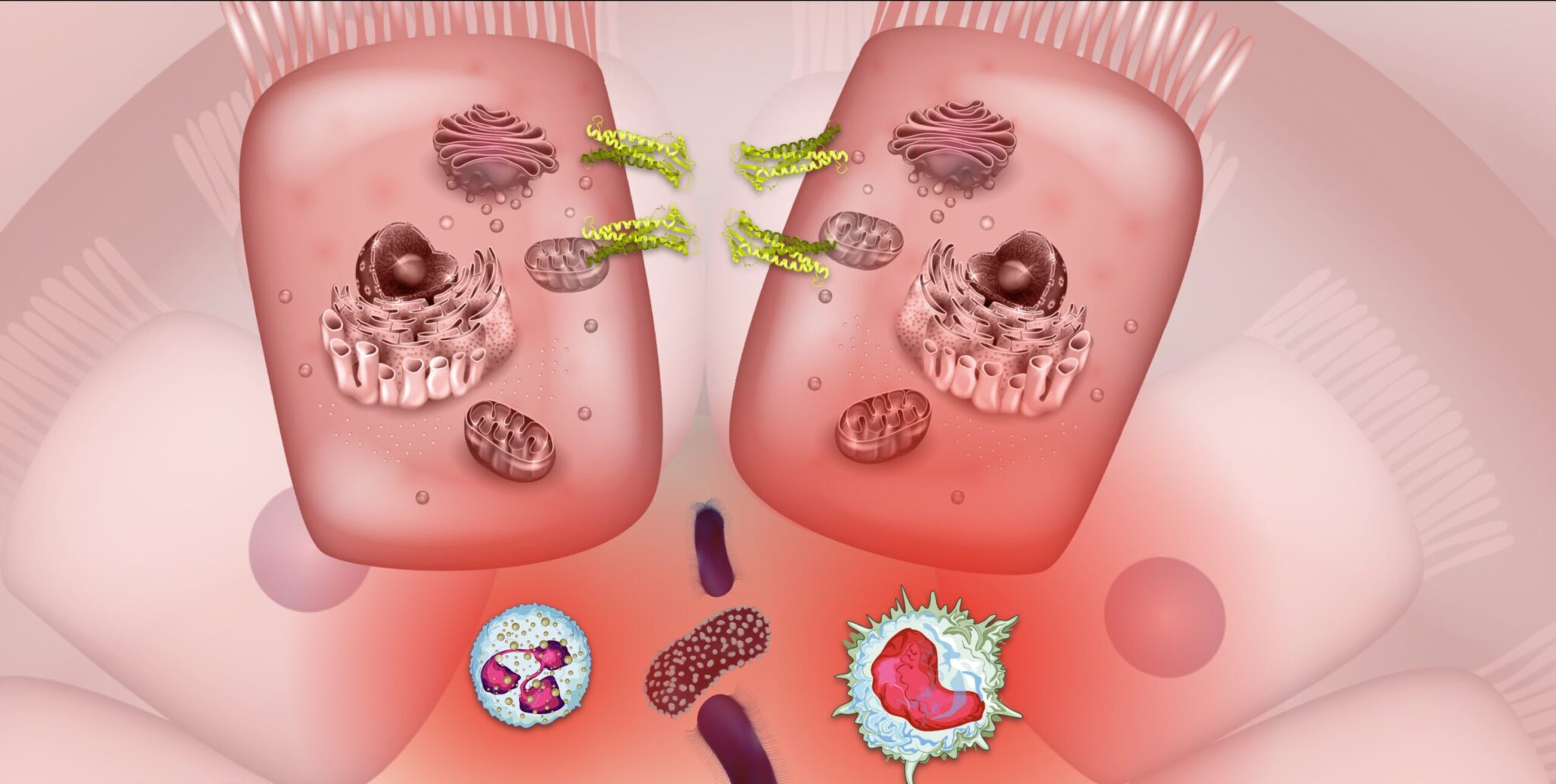
Inflammatory bowel disease is historically considered an autoimmune disease but is now better understood to be an immune-mediated condition. The hallmark pathology of IBD is inflammation in the GI tract that results in a major inflammatory response.20 This inflammatory response cycle is responsible for many of the symptoms of IBD and can fuel disease activity and progression. Increased intestinal barrier permeability and subsequent leaking of unwanted particles and bacteria across the gut lining is frequently observed in inflammatory bowel disease. This leaky gut barrier may relate strongly to dysbiosis in the gut microbiome and disruptions to serotonin signaling. As bacterial organisms and other luminal particulates leak across the epithelial membrane into the lamina propria, the immune system mounts a powerful response involving multiple cytokines like TNF and white blood cells like macrophages and neutrophils. This immune response, in an attempt to eliminate the invading threat, leads to heightened inflammation, increased intestinal permeability, and disruptions to the gut microbiome.
The Microbiome-Gut-Brain Axis in IBD
Scientific research on risk factors for IBD has consistently demonstrated a link between the gut, the microbiome, and IBD outcomes.21 In fact, people with inflammatory bowel disease have unique microbiome profiles when compared to people without IBD. The population of microbes living in the human gut may be influenced by factors such as stress, diet, and even psychedelics, which could in turn influence IBD symptoms and outcomes. This complex relationship between the gut microbiome and IBD is bidirectional: environmental factors like stress, diet, and psychedelics may impact the gut microbiome and subsequent IBD symptoms, but the increasing presence of symptoms, inflammation, and microbial dysbiosis may also impact things like dietary choices, stress, and the brain.
Learn More About The Microbiome-Gut-Brain Axis & IBD
The Brain and Neuroplasticity in IBD
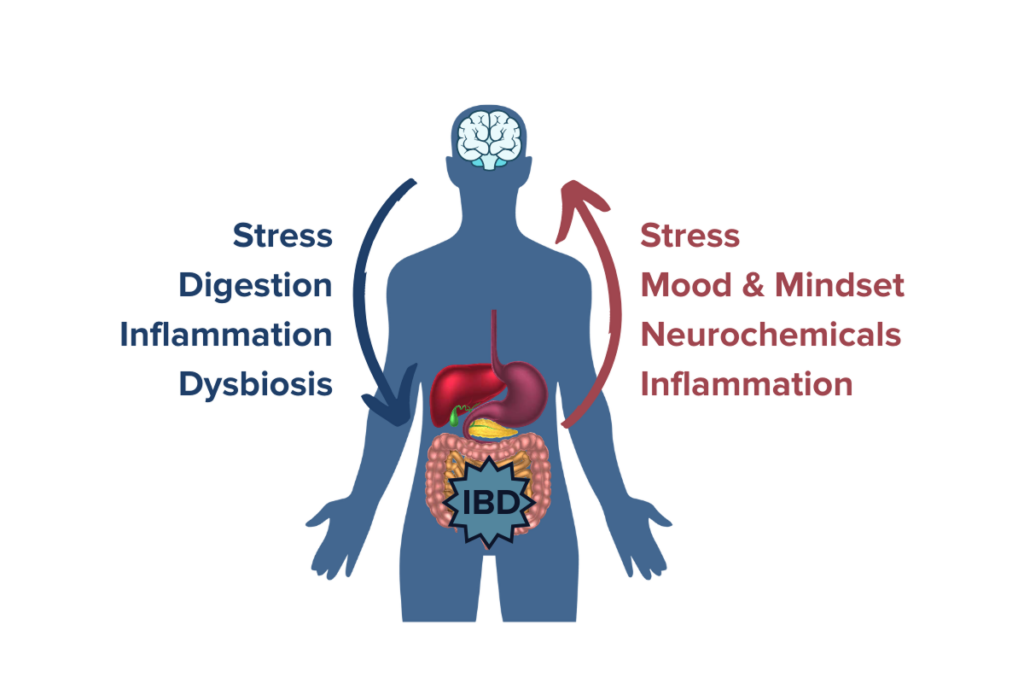
Through the microbiome-gut-brain axis, both physical and psychological stress have been linked to increased intestinal permeability, one of the primary mechanisms of IBD.22 Studies confirm that psychological stress increases symptom prevalence and exacerbation in IBD by disturbing the intestinal microbiota and triggering an immune system response. 23,24 In fact, people with IBD tend to have higher rates of psychological abnormalities compared to people with other chronic diseases.25 Other studies have also shown that people who have experienced childhood trauma or have PTSD also have a higher risk of developing autoimmune conditions.26 These studies highlight the importance of neuroplasticity in managing a condition like IBD because traumatic events, chronic stress, or conditions like depression and anxiety are known to induce lasting changes in brain circuitry that can have negative downstream effects like making stress systems in the brain even more sensitive. Managing stress levels and leveraging the power of neuroplasticity to enact positive change on the activity of the microbiome-gut-brain axis may be promising avenues by which people with IBD can positively impact their gut and brain health. Changes in the brain and neuroplasticity are likely some of the strongest driving forces behind the therapeutic potential of psychedelics for IBD.
Therapeutic Potential of Psychedelics in Inflammatory Bowel Disease
Recent research suggests that psychedelics like LSD and psilocybin (found in magic mushrooms) can help with depression, anxiety, and addiction. But could they also help people with inflammatory disorders including IBD? Most psychedelics exert their primary effects by binding to serotonin receptors in the brain, though they may also interact with gut serotonin systems. The gut harbors vast amounts of serotonin and also contains plentiful 5-HT2A receptors (the receptors to which psychedelic compounds like psilocybin preferentially bind). Serotonin in the gut has been associated with inflammation and changes in the gut microbiome, so psychedelics may be able to modulate serotonin signaling in the gut and induce beneficial effects on inflammation and the microbiome.
Psychoneuroimmunology, Psychedelics, and IBD
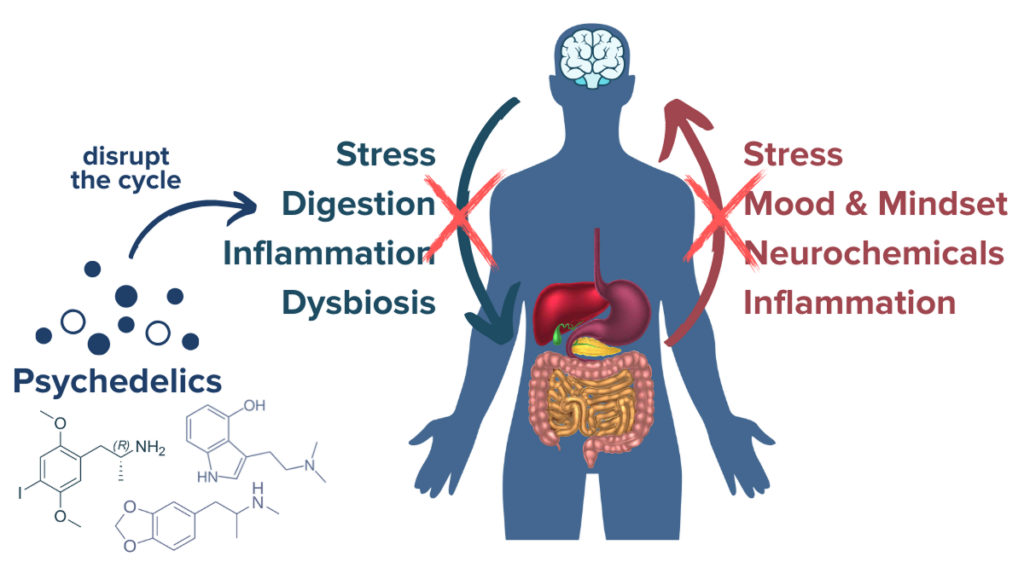
Psychoneuroimmunology is the field of research that investigates the relationship between the immune system, endocrine system, and nervous system. It is through these complex systems that psychedelics may exert many of their beneficial effects for immune-mediated conditions like IBD. One promising recent study demonstrated a direct link between psychedelics and gut inflammation. The phenethylamine called DOI possesses super-potent immunomodulatory qualities that include reductions in inflammatory pathways like NF-κB and TNF-α, both of which are deeply associated with IBD, with researchers noting that “psychedelics produce a potent blockade of the inflammation produced by TNF-α in cell and animal models of inflammation.”27 In an animal model of colitis, ketamine administration was able to reduce inflammatory signals like TNF-α in the colon.28 Some of the current most effective pharmaceutical treatments for inflammatory bowel disease work by disrupting inflammation via the NF-κB and TNF-α inflammatory pathways. These inflammatory signaling pathways are modulated by serotonin, which is known to be sensitive to both psychedelics and disturbances in the gut microbiome such as those seen in IBD. The recent finding that psychedelics can result in a potent blockade of NF-κB and TNF-α pathways to reduce inflammation stem from animal models, which are not a perfect representation of humans with inflammatory bowel disease.29 These studies do, however, help confirm the potential pathways by which psychedelics may act therapeutically in IBD and other inflammatory conditions and pave the way for further research in the future. Beyond influencing inflammation in the gut, psychedelics may also impart beneficial effects on people with inflammatory bowel disease by interacting with the gut-brain-microbiome axis and influencing neuroplasticity.
Gut-Brain-Microbiome Axis, Neuroplasticity, and Psychedelics for IBD
Beyond the role that psychedelics may play in modulating gut serotonin systems and inflammation in the GI tract, there may be potential benefits of psychedelics for IBD that relate directly to changes in neuroplasticity. While studies have not been performed directly on the relationship between neuroplasticity, psychedelics, and IBD, studies on the link between other chronic conditions and mental health conditions provide interesting insights into these potential therapeutic benefits.
The Link Between Neuroplasticity, Psychedelics, the Microbiome and IBD
Studies suggest neuroplasticity is the common mechanism by which different psychedelics have beneficial effects on conditions like depression.15 Recent studies have shown that psilocybin can stimulate rapid, lasting structural remodeling in the cortex of mice and alleviated stress-related behavioral deficits. This remodeling consisted of dendritic spine growth (an increase in neural connectivity) that lasted for over a month following the administration of psilocybin.19 Dendritic spines are a portion of the neuron that resemble the arbor of a tree and are used to capture signals sent from other neurons. Growth in dendritic spines indicates an increased potential for functional connectivity with other neurons. Additionally, many psychedelics have been observed to increase the expression of BDNF, a protein associated with neural growth.3,12 If psychedelics can enhance neuroplasticity and promote lasting, positive changes in mood and mindset, they could have significant benefits for some individuals with IBD. These potential benefits may be especially potent for those who also suffer from considerations like PTSD, depression, or anxiety alongside their IBD.
Some of these benefits may also be due in part to changes in the gut microbiome. Animal studies of ketamine therapy have demonstrated that the anti-depressant and anti-inflammatory effects of ketamine were accompanied by amplification of beneficial gut microbes like Lactobacillus and a decrease in potentially pathogenic microbes like Mucispirillum and Ruminococcus.30 Additionally, psychedelics like ayahuasca have been observed to have some antimicrobial properties, furthering the speculation that psychedelics may directly influence the composition of the intestinal microbiota.3 It is also well understood that serotonin and treatment with the serotonin precursor tryptophan can influence microbiome composition. Considering that most psychedelics bear structural similarity to the serotonin and tryptophan modules, it becomes clearer how they may be able to influence gut microbes. Whether psychedelics can impart lasting changes in gut ecology remains to be studied in humans, but still, this growing body of evidence highlights many mechanisms by which psychedelics may help IBD by influencing the gut, inflammation, neuroplasticity, and serotonin systems throughout the body.
Safely Using Psychedelics as Medicine
Psychedelics are not a part of everyone’s journey. For some individuals, using psychedelics therapeutically to influence neuroplasticity may be a valuable tool for their IBD protocol. For others, psychedelics may introduce unwelcomed volatility to the mind, brain, and body. The evidence described above regarding the benefits of psychedelic use all come from psychedelics administered in a controlled and safe research lab or therapeutic settings and it is not yet fully understood if these benefits persist in recreational or other settings. In clinical settings, psychedelics like MDMA and psilocybin have been demonstrated to be safe for use under the supervision of professionals, but once again this may not extend to psychedelic use in unsupervised or recreation settings. Understanding the potential consequences and adverse effects of psychedelics is essential to determine if they may be beneficial to someone’s IBD journey. Only you can ultimately decide whether or not psychedelics may be a good fit for you, and it is important to consult with your healthcare providers before adding any new substances to your IBD protocol.
The Legality of Therapeutic Psychedelics
Laws regarding the use of psychedelics vary by country, state, and municipality and also vary from substance to substance.35 While there are several ongoing clinical trials for conditions like depression and PTSD with various psychedelic therapeutics, psychedelics are not currently legal for widespread use. Additionally, there have not yet been studies investigating the specific effects of psychedelics like magic mushrooms for IBD. If you are hoping to pursue psychedelics as a part of your IBD journey, it is important to understand your local laws and regulations.
Safe Sources, Guides, and Licensed Therapists
When it comes to using psychedelics medicinally for symptoms of inflammatory bowel disease, it is important to keep safety in mind. Psychedelics obtained from unofficial sources run the risk of being contaminated with other illicit substances or psychoactive compounds that may have unwanted and even dangerous effects. (For more information about the risks associated with substances of unknown origins, DanceSafe and other harm reduction organizations produce educational materials and at-home adulterant test kits.) A growing number of treatment centers and trained guides are approved to safely study and administer medicinal psychedelics, such as the Heffter Foundation. Research centers have safe access to controlled supplies of psychoactive compounds like psilocybin mushrooms and MDMA. Additionally, many clinics that provide therapeutic psychedelic sessions offer guides or licensed therapists to accompany patients through the treatment process and ensure patients have a positive, safe, and therapeutic experience. Set, setting and mindset can dramatically influence the outcome of a psychedelic treatment session, so it is essential to be in a safe and positive environment. These factors are likely why adverse effects of psychedelics have seldom been observed in clinical and research studies. However, it is still important to be aware of the adverse psychological and physiological effects of psychedelic compounds that have been reported in recreational and uncontrolled settings.
Adverse Effects of Psychedelics
Psychological Effects
Some studies and reports have found that psychedelics (used outside of clinical, supervised settings) may have unintended side effects such as feelings of paranoia, psychosis, and panic that in some cases may even cause or worsen a psychological disorder.31 A 2015 publication from Nature, however, suggests that psychedelics do not increase the risk of mental health conditions and previous evidence may simply be a misinterpreted correlation–over 10% of adults in the US have tried psychedelics before, and mental health disorders are also highly prevalent in the general population, so there is expected overlap between people who have tried psychedelics and people who already have an existing mental health condition.32 This highlights the importance, however, of consuming psychedelics in a safe and supervised clinical setting where these adverse effects have not been observed in clinical trials.
Physiological Effects
Side effects that are commonly reported with psychedelic use outside of clinical settings include nausea (especially when orally consuming a psychedelic such as psilocybin mushrooms), increased heart rate, increased body temperature, loss of coordination, dry mouth, and sleep disturbances that may be especially uncomfortable for someone actively experiencing symptoms of IBD. This highlights the value of seeking a safe setting and professional supervision if you are considering psychedelic use to help monitor for and minimize the risk of any physiological side effects.
In recreational settings, things like dose, setting, and conflicting factors may contribute to adverse side effects. Most psychedelics act by interacting with the serotonin system in the brain and body. When taken in high doses or when combined with other medications that increase serotonin, psychedelics like MDMA can result in something known as serotonin syndrome where too much serotonin builds up in the body. Monoamine oxidase inhibitors (found naturally in ayahuasca or as a pharmacological medication for depression) also increase serotonin levels and can increase the risk of serotonin syndrome when consuming other psychedelics. Symptoms of serotonin syndrome range from mild (agitation, restlessness, high blood pressure, and rapid heartbeat) to severe (high fever, irregular heartbeat, and loss of consciousness).33 If signs or symptoms of serotonin syndrome are present seek medical attention immediately. High doses of ketamine can result in something colloquially known as “k-holing” where an individual becomes so impaired they temporarily cannot interact with the environment around them because they feel entirely separated from their body.34 It is essential to take psychedelics in a safe setting and understand what is present in the substance (and anything else it is being taken with) to avoid these potentially dangerous side effects.3
The Future of Medicine and Science Might be More Trippy Than We Think
Emerging evidence strongly suggests that psychedelics act as catalysts for change in both the brain and body by influencing neuroplasticity and serotonin systems. It is through these mechanisms that psychedelics may have potential therapeutic benefits for human conditions ranging from depression to autoimmune disorders. The evidence behind the benefits of psychedelics for some conditions is so strong that MDMA and psilocybin mushrooms have been declared Breakthrough Therapies for PTSD and treatment-resistant depression, respectively. It is through similar mechanisms of action that psychedelics for IBD may someday become an available therapeutic option. While the research is still young, psychedelics may have a two-fold benefit against IBD by directly reducing inflammatory pathways in the GI tract and improving neuroplasticity to enact lasting positive effects in the gut-brian-microbiome axis. Crohn’s disease and ulcerative colitis are known to relate to a complex system involving genetic and environmental risk factors fueling a perfect storm, and it appears that psychedelics may disrupt several mechanisms of the IBD cycle to promote healing.
While we may still be many years off from seeing studies that directly investigate psychedelics treatment options for IBD, it seems increasingly likely that psychedelics like psilocybin “magic mushrooms” will be formally approved for some conditions within the next two years. Seeing clinical trials succeeding for conditions like PTSD and depression will open the door for further study in other related conditions like inflammatory bowel disease. Could IBD be the next condition for which magic mushrooms are approved for use?
- Loddo I, Romano C. Inflammatory Bowel Disease: Genetics, Epigenetics, and Pathogenesis. Front Immunol. 2015;6:551. doi:10.3389/fimmu.2015.00551
- Günther C, Rothhammer V, Karow M, Neurath M, Winner B. The Gut-Brain Axis in Inflammatory Bowel Disease—Current and Future Perspectives. Int J Mol Sci. 2021;22(16):8870. doi:10.3390/ijms22168870
- Thompson C, Szabo A. Psychedelics as a novel approach to treating autoimmune conditions. Immunol Lett. 2020;228:45-54. doi:10.1016/j.imlet.2020.10.001
- Doblin RE, Christiansen M, Jerome L, Burge B. The Past and Future of Psychedelic Science: An Introduction to This Issue. J Psychoactive Drugs. 2019;51(2):93-97. doi:10.1080/02791072.2019.1606472
- Future Research. Heffter Research. Accessed February 27, 2022. https://www.heffter.org/future-research/
- Our Research. Multidisciplinary Association for Psychedelic Studies – MAPS. Accessed February 27, 2022. https://maps.org/our-research/
- Research. Usona Institute. Accessed February 27, 2022. https://www.usonainstitute.org/research/
- About psilocybin therapy : Compass Pathways. https://compasspathways.com/. Accessed February 27, 2022. https://compasspathways.com/our-research/psilocybin-therapy/about-psilocybin-therapy/
- Feduccia AA, Jerome L, Yazar-Klosinski B, Emerson A, Mithoefer MC, Doblin R. Breakthrough for Trauma Treatment: Safety and Efficacy of MDMA-Assisted Psychotherapy Compared to Paroxetine and Sertraline. Front Psychiatry. 2019;10:650. doi:10.3389/fpsyt.2019.00650
- Saplakoglu Y. FDA Calls Psychedelic Psilocybin a “Breakthrough Therapy” for Severe Depression. livescience.com. Published November 25, 2019. Accessed February 27, 2022. https://www.livescience.com/psilocybin-depression-breakthrough-therapy.html
- neurotransmitter | Definition, Signaling, & Types | Britannica. Accessed February 27, 2022. https://www.britannica.com/science/neurotransmitter
- de Vos CMH, Mason NL, Kuypers KPC. Psychedelics and Neuroplasticity: A Systematic Review Unraveling the Biological Underpinnings of Psychedelics. Front Psychiatry. 2021;12. Accessed February 27, 2022. https://www.frontiersin.org/article/10.3389/fpsyt.2021.724606
- Manocha M, Khan WI. Serotonin and GI Disorders: An Update on Clinical and Experimental Studies. Clin Transl Gastroenterol. 2012;3(4):e13. doi:10.1038/ctg.2012.8
- Kwon YH, Wang H, Denou E, et al. Modulation of Gut Microbiota Composition by Serotonin Signaling Influences Intestinal Immune Response and Susceptibility to Colitis. Cell Mol Gastroenterol Hepatol. 2019;7(4):709-728. doi:10.1016/j.jcmgh.2019.01.004
- Aleksandrova LR, Phillips AG. Neuroplasticity as a convergent mechanism of ketamine and classical psychedelics. Trends Pharmacol Sci. 2021;42(11):929-942. doi:10.1016/j.tips.2021.08.003
- Castelhano J, Lima G, Teixeira M, Soares C, Pais M, Castelo-Branco M. The Effects of Tryptamine Psychedelics in the Brain: A meta-Analysis of Functional and Review of Molecular Imaging Studies. Front Pharmacol. 2021;12. Accessed February 27, 2022. https://www.frontiersin.org/article/10.3389/fphar.2021.739053
- Fadiman J, Kornfeld A. Psychedelic‐Induced Experiences. In: Friedman HL, Hartelius G, eds. The Wiley‐Blackwell Handbook of Transpersonal Psychology. 1st ed. Wiley; 2013:352-366. doi:10.1002/9781118591277.ch19
- Iravani MM, Asari D, Patel J, Wieczorek WJ, Kruk ZL. Direct effects of 3,4-methylenedioxymethamphetamine (MDMA) on serotonin or dopamine release and uptake in the caudate putamen, nucleus accumbens, substantia nigra pars reticulata, and the dorsal raphé nucleus slices. Synap N Y N. 2000;36(4):275-285. doi:10.1002/(SICI)1098-2396(20000615)36:4<275::AID-SYN4>3.0.CO;2-#
- Shao LX, Liao C, Gregg I, et al. Psilocybin induces rapid and persistent growth of dendritic spines in frontal cortex in vivo. Neuron. 2021;109(16):2535-2544.e4. doi:10.1016/j.neuron.2021.06.008
- Ramos GP, Papadakis KA. Mechanisms of Disease: Inflammatory Bowel Diseases. Mayo Clin Proc. 2019;94(1):155-165. doi:10.1016/j.mayocp.2018.09.013
- Zuo T, Ng SC. The Gut Microbiota in the Pathogenesis and Therapeutics of Inflammatory Bowel Disease. Front Microbiol. 2018;9. Accessed February 27, 2022. https://www.frontiersin.org/article/10.3389/fmicb.2018.02247
- Kelly JR, Kennedy PJ, Cryan JF, Dinan TG, Clarke G, Hyland NP. Breaking down the barriers: the gut microbiome, intestinal permeability and stress-related psychiatric disorders. Front Cell Neurosci. 2015;9:392. doi:10.3389/fncel.2015.00392
- Mawdsley JE, Rampton DS. Psychological stress in IBD: new insights into pathogenic and therapeutic implications. Gut. 2005;54(10):1481-1491. doi:10.1136/gut.2005.064261
- Gao X, Cao Q, Cheng Y, et al. Chronic stress promotes colitis by disturbing the gut microbiota and triggering immune system response. Proc Natl Acad Sci U S A. 2018;115(13):E2960-E2969. doi:10.1073/pnas.1720696115
- Mental Health—An invisible driver of IBD cost of care. Crohn’s & Colitis Foundation. Accessed February 27, 2022. https://www.crohnscolitisfoundation.org/blog/mental-health-invisible-driver-ibd-cost-care
- Dube SR, Fairweather D, Pearson WS, Felitti VJ, Anda RF, Croft JB. Cumulative Childhood Stress and Autoimmune Diseases in Adults. Psychosom Med. 2009;71(2):243-250. doi:10.1097/PSY.0b013e3181907888
- Flanagan TW, Nichols CD. Psychedelics as anti-inflammatory agents. Int Rev Psychiatry. 2018;30(4):363-375. doi:10.1080/09540261.2018.1481827
- Ashry EE, Abdellatief RB, Mohamed AE, Kotb HI. Protective Effect of Ketamine against Acetic Acid-Induced Ulcerative Colitis in Rats. Pharmacol Amp Pharm. 2016;07(01):9-18. doi:10.4236/pp.2016.71002
- Bischoff SC, Mailer R, Pabst O, et al. Role of serotonin in intestinal inflammation: knockout of serotonin reuptake transporter exacerbates 2,4,6-trinitrobenzene sulfonic acid colitis in mice. Am J Physiol-Gastrointest Liver Physiol. 2009;296(3):G685-G695. doi:10.1152/ajpgi.90685.2008
- Getachew B, Aubee JI, Schottenfeld RS, Csoka AB, Thompson KM, Tizabi Y. Ketamine interactions with gut-microbiota in rats: relevance to its antidepressant and anti-inflammatory properties. BMC Microbiol. 2018;18:222. doi:10.1186/s12866-018-1373-7
- Hallucinogens DrugFacts. National Institute on Drug Abuse. Published April 22, 2019. Accessed February 27, 2022. https://nida.nih.gov/publications/drugfacts/hallucinogens
- Cormier Z. No link found between psychedelics and psychosis. Nature. Published online March 4, 2015. doi:10.1038/nature.2015.16968
- Serotonin syndrome – Symptoms and causes. Mayo Clinic. Accessed February 27, 2022. https://www.mayoclinic.org/diseases-conditions/serotonin-syndrome/symptoms-causes/syc-20354758
- K-Hole: What It Feels Like, Safety, and More. Healthline. Published October 9, 2019. Accessed February 27, 2022. https://www.healthline.com/health/k-hole
- Drug Scheduling. Accessed February 27, 2022. https://www.dea.gov/drug-information/drug-scheduling